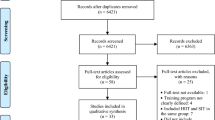
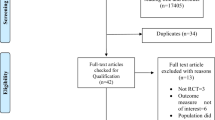
Abstract
Background
Some health benefits from high-intensity interval training (HIIT) are facilitated by peripheral blood lactate levels. However, the lactate response from HIIT is variable and dependent on protocol parameters.
Objectives
We aimed to determine the HIIT protocol parameters that elicited peak lactate levels, and how these levels are associated with post-HIIT cognitive performance.
Study Design
We conducted a systematic review with meta-regression.
Methods
MEDLINE, Embase, CENTRAL, SPORTDiscus, and CINAHL + were searched from database inception to 8 April, 2022. Peer-reviewed primary research in healthy adults that determined lactate (mmol/L) and cognitive performance after one HIIT session was included. Mixed-effects meta-regressions determined the protocol parameters that elicited peak lactate levels, and linear regressions modelled the relationship between lactate levels and cognitive performance.
Results
Study entries (n = 226) involving 2560 participants (mean age 24.1 ± 4.7 years) were included in the meta-regression. A low total work-interval volume (~ 5 min), recovery intervals that are about five times longer than work intervals, and a medium session volume (~ 15 min), elicited peak lactate levels, even when controlling for intensity, fitness (peak oxygen consumption) and blood measurement methods. Lactate levels immediately post-HIIT explained 14–17% of variance in Stroop interference condition at 30 min post-HIIT.
Conclusions
A HIIT protocol that uses the above parameters (e.g., 8 × 30-s maximal intensity with 90-s recovery) can elicit peak lactate, a molecule that is known to benefit the central nervous system and be involved in exercise training adaptations. This review reports the state of the science in regard to the lactate response following HIIT, which is relevant to those in the sports medicine field designing HIIT training programs.
Trial Registry
Clinical Trial Registration: PROSPERO (CRD42020204400).







Similar content being viewed by others
References
Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle. Part I: cardiopulmonary emphasis. Sports Med. 2013;43(5):313–38.
Laursen PB. Training for intense exercise performance: high-intensity or high-volume training? Scand J Med Sci Sports. 2010;20(Suppl 2):1–10.
Milanovic Z, Sporis G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–81.
Hwang CL, Yoo JK, Kim HK, et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–9.
Maillard F, Rousset S, Pereira B, et al. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab. 2016;42(6):433–41.
Gillen JB, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl Physiol Nutr Metab. 2014;39(3):409–12.
Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle. Part II: anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013;43(10):927–54.
Beneke R, Leithauser RM, Ochentel O. Blood lactate diagnostics in exercise testing and training. Int J Sports Physiol Perform. 2011;6(1):8–24.
Falz R, Fikenzer S, Holzer R, et al. Acute cardiopulmonary responses to strength training, high-intensity interval training and moderate-intensity continuous training. Eur J Appl Physiol. 2019;119(7):1513–23.
Fletcher WM. Lactic acid in amphibian muscle. J Physiol. 1907;35(4):247–309.
Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558(Pt 1):5–30.
Proia P, Di Liegro CM, Schiera G, et al. Lactate as a metabolite and a regulator in the central nervous system. Int J Mol Sci. 2016;17(9):1450.
Adeva-Andany M, Lopez-Ojen M, Funcasta-Calderon R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76–100.
Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27(4):757–85.
Bergman BC, Tsvetkova T, Lowes B, et al. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol. 2009;587(Pt 9):2087–99.
Emhoff CA, Messonnier LA, Horning MA, et al. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol. (1985). 2013;114(3):297–306.
Glenn TC, Martin NA, Horning MA, et al. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma. 2015;32(11):820–32.
Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;22(10):3443–9.
van Hall G, Stromstad M, Rasmussen P, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29(6):1121–9.
Morland C, Andersson KA, Haugen OP, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun. 2017;8:15557.
Muller P, Duderstadt Y, Lessmann V, et al. Lactate and BDNF: key mediators of exercise induced neuroplasticity? J Clin Med. 2020;9(4):1136.
Ichihara Y, Doi T, Ryu Y, et al. Oligodendrocyte progenitor cells directly utilize lactate for promoting cell cycling and differentiation. J Cell Physiol. 2017;232(5):986–95.
Sanchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 2001;36(3):321–9.
Matsui T, Omuro H, Liu YF, et al. Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. Proc Natl Acad Sci USA. 2017;114(24):6358–63.
Alvarez Z, Castano O, Castells AA, et al. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials. 2014;35(17):4769–81.
Zhou J, Liu T, Guo H, et al. Lactate potentiates angiogenesis and neurogenesis in experimental intracerebral hemorrhage. Exp Mol Med. 2018;50(7):1–12.
Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A, et al. L-lactate promotes adult hippocampal neurogenesis. Front Neurosci. 2019;13:403.
Hashimoto T, Hussien R, Oommen S, et al. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21(10):2602–12.
Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90.
Popov LD. Mitochondrial biogenesis: an update. J Cell Mol Med. 2020;24(9):4892–9.
Li PA, Hou X, Hao S. Mitochondrial biogenesis in neurodegeneration. J Neurosci Res. 2017;95(10):2025–9.
Nalbandian M, Takeda M. Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology (Basel). 2016;5(4):38.
Percival ME, Martin BJ, Gillen JB, et al. Sodium bicarbonate ingestion augments the increase in PGC-1alpha mRNA expression during recovery from intense interval exercise in human skeletal muscle. J Appl Physiol. (1985). 2015;119(11):1303–12.
Hoshino D, Tamura T, Masuda H, et al. Effects of decreased lactate accumulation after dichloroacetate administration on exercise training-induced mitochondrial adaptations in mouse skeletal muscle. Physiol Rep. 2015;3(9):e12555.
Yang J, Ruchti E, Petit JM, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci USA. 2014;111(33):12228–33.
Lezi E, Lu J, Selfridge JE, et al. Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem. 2013;127(1):91–100.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–64.
Coco M, Caggia S, Musumeci G, et al. Sodium L-lactate differently affects brain-derived neurothrophic factor, inducible nitric oxide synthase, and heat shock protein 70 kDa production in human astrocytes and SH-SY5Y cultures. J Neurosci Res. 2013;91(2):313–20.
Kujach S, Olek RA, Byun K, et al. Acute sprint interval exercise increases both cognitive functions and peripheral neurotrophic factors in humans: the possible involvement of lactate. Front Neurosci. 2020;13:1455.
Petersen AA, Larsen KE, Behr GG, et al. Brain-derived neurotrophic factor inhibits apoptosis and dopamine-induced free radical production in striatal neurons but does not prevent cell death. Brain Res Bull. 2001;56(3–4):331–5.
Hsieh SS, Chueh TY, Huang CJ, et al. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J Sports Sci. 2021;39(1):10–22.
Ai JY, Chen FT, Hsieh SS, et al. The effect of acute high-intensity interval training on executive function: a systematic review. Int J Environ Res Public Health. 2021;18(7):3593.
Hashimoto T, Tsukamoto H, Tsukamoto S, et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018;32(3):1417–27.
Tsukamoto H, Suga T, Takenaka S, et al. Repeated high-intensity interval exercise shortens the positive effect on executive function during post-exercise recovery in healthy young males. Physiol Behav. 2016;160:26–34.
Damrongthai C, Kuwamizu R, Suwabe K, et al. Benefit of human moderate running boosting mood and executive function coinciding with bilateral prefrontal activation. Sci Rep. 2021;11(1):22657.
Byun K, Hyodo K, Suwabe K, et al. Positive effect of acute mild exercise on executive function via arousal-related prefrontal activations: an fNIRS study. Neuroimage. 2014;98:336–45.
Kemppainen J, Aalto S, Fujimoto T, et al. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568(Pt 1):323–32.
Schiffer T, Schulte S, Sperlich B, et al. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett. 2011;488(3):234–7.
Tsukamoto H, Suga T, Takenaka S, et al. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol Behav. 2016;155:224–30.
Rodriguez AL, Whitehurst M, Fico BG, et al. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp Biol Med (Maywood). 2018;243(14):1153–60.
Boyne P, Meyrose C, Westover J, et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. J Appl Physiol. (1985). 2019;126(2):431–43.
Hashimoto T, Tsukamoto H, Ando S, et al. Effect of exercise on brain health: the potential role of lactate as a myokine. Metabolites. 2021;11(12):813.
El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–82.
Jacob N, So I, Sharma B, et al. Effects of high-intensity interval training on blood lactate levels and cognition in healthy adults: protocol for systematic review and network meta-analyses. Syst Rev. 2022;11(1):31.
Perianez JA, Lubrini G, Garcia-Gutierrez A, et al. Construct validity of the Stroop Color-Word Test: influence of speed of visual search, verbal fluency, working memory, cognitive flexibility, and conflict monitoring. Arch Clin Neuropsychol. 2021;36(1):99–111.
Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–68.
Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:I4919.
Brooks GA, Arevalo JA, Osmond AD, et al. Lactate in contemporary biology: a phoenix risen. J Physiol. 2021;600(5):1229–51.
Hargreaves M, Spriet LL. Exercise metabolism: fuels for the fire. Cold Spring Harb Perspect Med. 2018;8(8):a029744.
Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;2(9):817–28.
Morton AR. Chapter 8. Exercise physiology. In: Taussig LM, Landau LI, editors. Pediatric respiratory medicine. 2nd ed. Philadelphia: Mosby; 2008. p. 89–99.
Hyodo K, Dan I, Suwabe K, et al. Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol Aging. 2012;33(11):2621–32.
Yanagisawa H, Dan I, Tsuzuki D, et al. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50(4):1702–10.
Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35:101454.
Huang Z, Zhang Y, Zhou R, et al. Lactate as potential mediators for exercise-induced positive effects on neuroplasticity and cerebrovascular plasticity. Front Physiol. 2021;12:656455.
Chao D, Foy CG, Farmer D. Exercise adherence among older adults: challenges and strategies. Control Clin Trials. 2000;21(5 Suppl.):212S–S7.
El Ansari W, Lovell G. Barriers to exercise in younger and older non-exercising adult women: a cross sectional study in London, United Kingdom. Int J Environ Res Public Health. 2009;6(4):1443–55.
Tinker A, Molloy L, Monks I, et al. The benefits and barriers of exercise for the physical health of older women. J Aging Res Clin Pract. 2017;6:73–9.
Leal G, Bramham C, Duarte C. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153–95.
Stillman CM, Cohen J, Lehman ME, et al. Mediators of physical activity on neurocognitive function: a review at multiple levels of analysis. Front Hum Neurosci. 2016;10:626.
Xiao J, Wong AW, Willingham MM, et al. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18(3):186–202.
Lundgaard I, Luzhynskaya A, Stockley JH, et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013;11(12):e1001743.
Fields RD, Bukalo O. Myelin makes memories. Nat Neurosci. 2020;23(4):469–70.
Xin W, Chan JR. Myelin plasticity: sculpting circuits in learning and memory. Nat Rev Neurosci. 2020;21(12):682–94.
Poole DC, Rossiter HB, Brooks GA, et al. The anaerobic threshold: 50+ years of controversy. J Physiol. 2021;599(3):737–67.
Zhou L, Chen SY, Han HJ, et al. Lactate augments intramuscular triglyceride accumulation and mitochondrial biogenesis in rats. J Biol Regul Homeost Agents. 2021;35(1):105–15.
Park J, Kim J, Mikami T. Exercise-induced lactate release mediates mitochondrial biogenesis in the hippocampus of mice via monocarboxylate transporters. Front Physiol. 2021;12:736905.
Glancy B, Kane DA, Kavazis AN, et al. Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol. 2021;599(3):863–8.
Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab. 2006;290(6):e1237–44.
Hashimoto T, Hussien R, Cho HS, et al. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS ONE. 2008;3(8):e2915.
Messonnier LA, Emhoff CA, Emhoff JA, et al. Lactate kinetics at the lactate threshold in trained and untrained men. J Appl Physiol. (1985). 2013;114(11):1593–602.
Granata C, Jamnick NA, Bishop DJ. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 2018;48(8):1809–28.
Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2):162–84.
Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586(1):35–44.
Faude O, Kindermann W, Meyer T. Lactate threshold concepts: how valid are they? Sports Med. 2009;39(6):469–90.
Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–94.
Mann T, Lamberts RP, Lambert MI. Methods of prescribing relative exercise intensity: physiological and practical considerations. Sports Med. 2013;43(7):613–25.
Stark SM, Kirwan SB, Stark CEL. Mnemonic Similarity Task: a tool for assessing hippocampal integrity. Trends Cogn Sci. 2019;23(11):938–51.
Vieweg P, Riemer M, Berron D, et al. Memory image completion: establishing a task to behaviorally assess pattern completion in humans. Hippocampus. 2019;29(4):340–51.
Bekinschtein P, Oomen CA, Saksida LM, et al. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22(5):536–42.
Finnegan R, Becker S. Neurogenesis paradoxically decreases both pattern separation and memory interference. Front Syst Neurosci. 2015;9:136.
Becker S. Neurogenesis and pattern separation: time for a divorce. Wiley Interdiscipl Rev Cogn Sci. 2017;8(3):e1427.
Acknowledgements
The authors thank Jessica Babineau (Information Specialist with Library & Information Services at Toronto Rehabilitation Institute, University Health Network) for the helpful consultations regarding search strategy, screening, data extraction and risk of bias assessments. The authors also thank Dr. Mohammad Alavinia (Biostatistician III at Toronto Rehabilitation Institute, University Health Network) for the helpful consultations regarding the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This review is funded by the Canada Research Chairs Program and the Natural Sciences and Engineering Research Council of Canada Discovery Grant (UT458054) to the senior author. Donor funds were provided by the Walter and Maria Schroeder Family Foundation and by the Brain Changes Initiative.
Conflict of interest
Nithin Jacob, Isis So, Bhanu Sharma, Susan Marzolini, Maria Carmela Tartaglia, Paul Oh and Robin Green have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable as this article does not contain data from any individual person.
Availability of data and material
All data generated or analysed during this review are included in the published paper and supplementary material.
Code availability
Not applicable.
Author contributions
NJ conceived the study, completed all statistical analyses and wrote the first manuscript draft. NJ and IS independently screened titles, abstracts, and full texts, and extracted data. BS reviewed all conflicts during screening, and provided valuable guidance during data extraction and analyses. IS, BS, SM, CT, PO and RG critically edited and revised the draft manuscript, and provided input throughout the review process. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jacob, N., So, I., Sharma, B. et al. Effects of High-Intensity Interval Training Protocols on Blood Lactate Levels and Cognition in Healthy Adults: Systematic Review and Meta-Regression. Sports Med 53, 977–991 (2023). https://doi.org/10.1007/s40279-023-01815-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-023-01815-2