
Background and Overview
Type 1 diabetes (T1D) is an autoimmune disease that ultimately results in the destruction of insulin-producing beta cells and a life-long dependence on carefully titrated exogenous insulin. It is estimated that approximately 1.6 million people in the US and around 20 million globally are affected by T1D.
JDRF’s mission is to accelerate the delivery of life-changing breakthroughs that prevent, cure and treat T1D and its complications. We have come a long way toward this goal with the availability of automated glucose measurement and management systems, improved insulins and a promising pipeline of therapeutic candidates that have the potential to modify the course of the disease and reduce insulin dependence. However, there is still a long way to go.
Goals
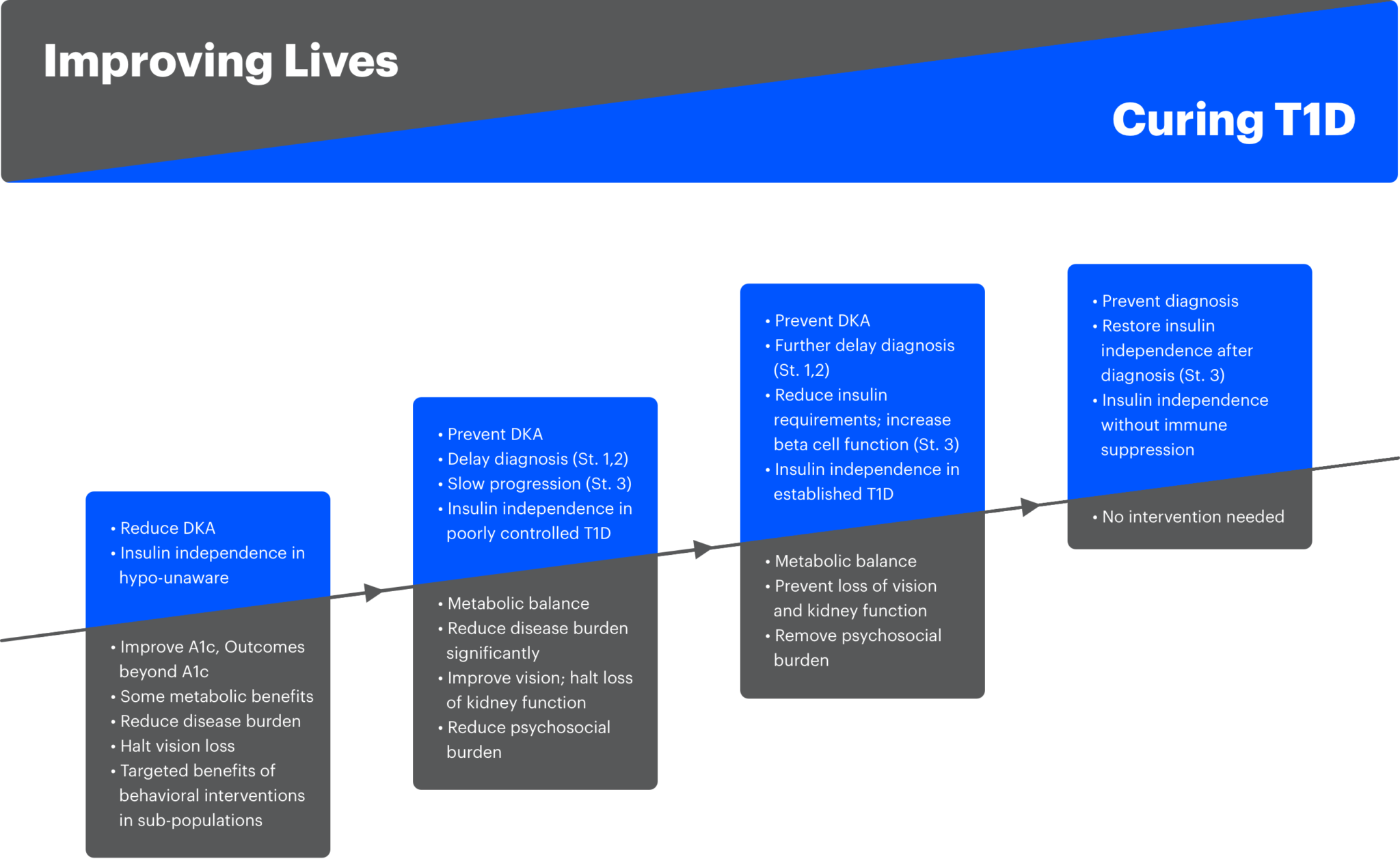
Our research strategy rests on two pillars: Cure T1D and Improve Lives of individuals with T1D. JDRF research will prioritize projects with the highest likelihood of accelerating the delivery of therapies to cure (and prevent) T1D while maintaining strategic gap-filling funding in research that improves lives of T1D, and will focus on the following over the next five years:
- Develop and execute a global universal screening strategy that reduces DKA at diagnosis, identifies high-risk individuals for early detection and accelerate the evaluation of disease-modifying therapies, identify individuals that will be eligible for approved preventive treatments, and in conjunction develop cost-benefit analyses of screening for payer assessment.
- Accelerate the development of disease modifying therapies that delay, stop or reverse the development and progression of T1D, enable clinical testing of these therapies, and facilitate development of business models to support T1D drug development and commercialization.
- Accelerate the development of first-generation beta cell replacement products demonstrating at least 6 months of reduction in insulin requirements, potentially with the use of local immunosuppression.
- Improve overall outcomes in people with T1D (and potentially a broader population of individuals with insulin-requiring diabetes), reduce disease burden and complications, and reduce barrier to adoption of newly approved life-changing breakthroughs.
- Foster a community of T1D researchers and clinicians through training awards and networking activities, and bridge the gap between endocrinologists, transplant surgeons, psychologists and immunologists.
Execution
The following guiding principles will support the accelerated delivery of products to change the lives of people with T1D:
- Enable progression of therapies through the R&D pipeline with a line of sight to product development and reduce the barriers in product development and commercialization throughout the process.
- Support innovation to fill knowledge gaps and create potential for novel treatment paradigms and product development.
- Make ‘big bets’ to aggressively drive new research opportunities and promising initiatives.
- Create and sustain partnerships with global funders and stakeholders, such as the National Institutes of Health, Helmsley Charitable Trust, other foundations and government funders that leverage resources and complement strengths to fill gaps and remove barriers.
- Cultivate multi-disciplinary teams (consortia, public-private projects, coalitions, others) to address specific scientific challenges.
- Influence via thought leadership to guide activities of T1D stakeholders (academic, private sector, government) to achieve our goals.

Summary
Success for JDRF is defined by the availability of affordable life changing therapies that improve the lives of people with or at risk of T1D. These products may also have the potential to impact broader populations, such as people with insulin requiring T2D or other autoimmune diseases. Success for the JDRF research and development pipeline depends on a diversified but balanced portfolio that includes academic and industry efforts that can successfully transition from early discovery to clinical trials and product development. JDRF allocates funding and non-funding resources to ensure clinical impact and accelerated therapeutic advancements by funding projects ranging from those that are relatively close to the clinic to those that are earlier in development, such as efforts in discovery and preclinical development with potential for significant advancement and impact in longer timeframes. Taken together, these will drive progress on our mission.

