Co-designing a Canadian adaptation of a lifestyle-oriented intervention aimed to improve daily functioning of individuals living with chronic pain: a multi-method study protocol of REVEAL(OT) Canada
- 1Research Center of the Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada
- 2Department of Biomedical Sciences, Faculty of Medicine, Université de Montréal, Montreal, QC, Canada
- 3The Research and Implementation Unit PROgrez, Department of Physiotherapy and Occupational Therapy, Næstved-Slagelse-Ringsted Hospitals, Slagelse, Denmark
- 4Department of Regional Health Research, University of Southern Denmark, Odense, Denmark
- 5User Perspectives and Community-Based Interventions, Department of Public Health, University of Southern Denmark, Odense, Denmark
- 6Research Unit of General Practice, Department of Public Health, University of Southern Denmark, Odense, Denmark
- 7Research Unit of General Practice, Aarhus University, Aarhus, Denmark
- 8DRIVEN, Department of Sports Science and Clinical Biomechanics, University of Southern Denmark, Odense, Denmark
- 9Center for Muscle and Joint Health, Department of Sports Science and Clinical Biomechanics, Faculty of Health, University of Southern Denmark, Odense, Denmark
- 10Faculty of Nursing, Université de Montréal, Montreal, QC, Canada
- 11Faculty of Medicine and Health Sciences, School of Physical and Occupational Therapy, McGill University, Montreal, QC, Canada
- 12Faculty of Medicine and Health Sciences, School of Rehabilitation, Université de Sherbrooke, Sherbrooke, QC, Canada
- 13Research Center of the Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC, Canada
- 14Department of Anesthesiology and Pain Medicine, Faculty of Medicine, Université de Montréal, Montreal, QC, Canada
- 15Department of Anesthesia, McGill University Health Centre, Montreal, QC, Canada
- 16Alan Edwards Pain Management Unit, Montreal General Hospital, Montreal, QC, Canada
- 17Patient Partner, Centre Hospitalier de l'Université de Montréal, Montreal, QC, Canada
- 18Department of Psychology, Faculty of Arts and Sciences, Université de Montréal, Montreal, QC, Canada
Background: Living with chronic pain (CP) often implies major lifestyle changes, including modifications of daily routines and work. Surprisingly, few validated and effective interventions specifically target functional outcomes in this population. Redesign your Everyday Activities and Lifestyle with Occupational Therapy [REVEAL(OT)] is a lifestyle-oriented intervention led by occupational therapists that directly targets the daily functional challenges of living with CP. The intervention was initially developed and studied as an add-on to standard treatment delivered by Danish multidisciplinary specialized pain clinics. Adapting, implementing, and evaluating REVEAL(OT) within the Canadian healthcare system will contribute to broadening the scope of treatments offered in specialized pain clinics that do not yet include occupational therapy.
Objective: The proposed study aims to define and refine REVEAL(OT)/CA with partners (authors of original intervention, people with lived experience, clinicians, managers).
Methods: This participatory action research will use a multi-method design and follow the ORBIT model for developing behavioral treatments for chronic diseases. A process of co-construction with partners and an advisory committee will take place in two Montreal specialized pain clinics. It consists of two related work packages (WPs). In WP1, a first series of focus groups with partners (n = 86) and workshops with the advisory committee will be conducted to co-develop the hypothetical pathway describing intervention components and their potential mechanisms of action on targeted outcomes, as well as the first version of the adapted intervention manual. WP2 will co-refine REVEAL(OT)/CA by exploring its acceptability, feasibility and mechanisms of action through intervention deliveries (at least twice in each of two specialized pain clinics; n ≥ 60 patients) and focus groups and/or individual interviews with participating patients and partners. At the end of this study, the intervention manual will be generated both in French and English.
Discussion: This study will set the stage for subsequent implementation and effectiveness assessment projects and be an important step towards the deployment of interventions aiming to improve engagement in meaningful daily activities among adults living with CP.
Registration: OSF Registries, osf.io/8gksa. Registered 3 August 2023, https://osf.io/8gksa.
Introduction
Chronic pain (CP), or pain that lasts more than 3 months (1), affects one in five Canadians (2), is associated with high disability (1, 3), and costs approximately CAN$40 billion each year when considering direct and indirect costs (4). CP frequently implies disruptions in their daily routines (5) and it is one of the chronic diseases associated with most years lived with disability (6) and occupational identity loss (7, 8). Despite the adverse human, societal, and economic consequences of CP (2, 9–13), it is often underdiagnosed and undertreated (14, 15). Biopsychosocial models (2, 4) are commonly endorsed theoretical understandings of the experience of pain, and multidisciplinary treatments are recognized as the gold standard for pain management (16, 17). Yet, current evidence does not portray a very optimistic picture, with most interventions only offering mild to moderate benefits (2, 4) and differential effects of treatment components being poorly documented (18). This can be illustrated by a recent study that showed that only 20% of individuals living with CP attending specialized pain clinics report clinically significant improvements in pain severity after 2 years (19).
In this context, recent reports from the Canadian Pain Task Force and the Action Plan for Pain in Canada highlight the need for innovative clinical models that can better treat or manage pain holistically (2, 4, 20). Individuals living with CP are concerned about the multiple occupational challenges they face on a day-to-day basis (8, 21, 22). Amongst 152 individuals living with musculoskeletal pain, 85% identified facing challenges associated with productivity (including paid/unpaid work, household management and play/school), 77% reported challenges associated with self-care (including all occupations related to personal care, functional mobility and community management) and 78% reported challenges associated with engaging in leisure activities (such as quiet recreation, active recreation and socialization) (23). These individuals believe that lifestyle is an important factor in health-related quality of life when living with CP and 92% of those entering a multidisciplinary pain treatment center report being motivated to change their lifestyle (24). Despite the high prevalence of occupational disturbances in this population and the need to address them, very few interventions within specialized pain clinics directly target occupational outcomes (9).
All these findings highlight the need for comprehensive programs promoting improved function and quality of life through occupational approaches (25, 26). According to the International Association for the Study of Pain [IASP] (27), occupational therapists are important members of specialized CP management clinics since they provide distinct value in the management of CP by directly targeting the person's engagement in significant occupations and by specifically using occupation itself as a medium for therapy (27–29). However, poor integration of occupational therapy within multidisciplinary care has been documented (30, 31) which might in part be due to a lack of training of occupational therapists in chronic pain management and poor visibility of the role occupational therapy can play in with this population (31). Lifestyle-oriented interventions, which put focus on facilitating client development and implementation of healthy routines and habits, have been studied recently (24, 32–34). It appears that, although pain intensity tends to remain statistically unchanged, engagement in meaningful activities was significantly improved, which is considered an important determinant of health, well-being and quality of life for individuals affected by CP (32, 34). Those interventions, however, were originally designed for chronic illnesses in general and were adapted to CP on a theoretical basis without involving collaborative process with partners. To our knowledge, very few experimental and quasi-experimental studies including occupational engagement in CP treatment for adult population are available.
Redesign your Everyday Activities and Lifestyle with Occupational Therapy [REVEAL(OT)], is an innovative intervention developed at the University of Southern Denmark and Region Zealand which has undergone three iteration phases to improve delivery, outcomes, and fit within specialized Danish pain clinics (24, 33, 35–37). Considering that several factors contributing to poorer mental and metabolic health and increased risks of chronicity (e.g., stress, physical activity, eating habits) are modifiable (38), addressing them could help break the vicious cycle of CP, poor functioning, and psychological distress. REVEAL(OT) proposes a combination of individual and group interventions over the course of 14 weeks. It is based on occupational therapy evidence about CP management through lifestyle changes, population-centered information on motivation for changing lifestyle, and contextual factors related to intervention delivery within a specialized tertiary care pain clinic. When added to usual care, REVEAL(OT) leads to satisfaction and improvement in a range of outcomes important to individuals living with CP, such as more effective activity pacing in real-life daily routines (33). A recent study has demonstrated REVEAL(OT) feasibility in a Danish pain clinic (35) by showing satisfactory program adherence and patients’ self-perceived relevance, timing and mode of delivery while retention and the fidelity of delivery needed improvement. This pre-post study detected a significant change in occupational performance and satisfaction (assessed by the Canadian Occupational Performance Measure, COPM). Change reached the minimal clinically important difference (MCID) for COPM performance in 14%, and for COPM satisfaction for 24% of the cases. Since it is specifically targeting occupational needs of individuals living with CP seen in specialized pain clinics (33), REVEAL(OT) was chosen for the current study over others with more effectiveness data but that were developed for other populations such as mental health (39, 40) or diabetes (41).
According to the Adapting Interventions to New Contexts (ADAPT) guidelines (42), it is often more efficient to adapt an existing intervention than to develop a new one. Identifying and exploring the impact of essential treatment components on targeted clinical outcomes is fundamental to the adaptation process (43). It is also crucial to involve partners since most health interventions are highly sensitive to the context in which they are delivered and it is important to ensure they meet the needs of the target population, they are deliverable by clinicians while considering organizational constraints (42). The proposed study aims to define and refine REVEAL(OT)/CA with partners (authors of original intervention, people with lived experience, clinicians, managers). This overall objective is operationalized in 4 specific objectives: (1) co-development of (a) the hypothetical pathway describing intervention components and their potential mechanisms of action on outcome and (b) the adapted intervention manual with partners (authors of original intervention, people with lived experience, clinicians, managers); (2) optimization of acceptability of intervention content and format, and feasibility of its delivery; (3) exploration of acceptability, feasibility and mechanisms of action of REVEAL(OT)/CA through initial delivery of the intervention; and (4) co-refinement of REVEAL(OT)/CA to generate a final manualized version prior to subsequent implementation and effectiveness assessment projects.
Methods
Overview
This participatory action research will use a multi-method study design to meet the stated objectives. Combining qualitative data about partners' appreciation of the intervention will drive outcomes used to explore its clinical effects, with an emphasis being placed on a democratic process in which all team members participate in research creation. An advisory committee will be formed of 2–3 patient-partners living with CP who have received care in specialized pain clinics, 2–3 clinicians from two pain clinics (coming from different professional backgrounds), 2 medical directors and 2 managers (at least 1 from each clinic), 3 international researchers which are also authors of REVEAL(OT) and 3 local researchers (with complementary research expertises). The composition of the committee may change over time depending on the expertise required, the availability of each member and the evolution of the project. The ORBIT model for developing behavioral treatments to prevent and/or manage chronic disease (43–46) will guide the methodology. This model, which is informed by the meticulous process of drug development, is based on a large consensus between renowed scientific committees including the Obesity-Related Behavioral Intervention Trials (ORBIT) consortium. It proposes a flexible and iterative progressive process, in which prespecified clinically significant milestones will guide forward or backward movements between each phase in order to develop an intervention and utimatly determine its effectiveness. The present study will carry out Phase Ia (Define the intervention) and Ib (Refine the intervention) of the ORBIT model.
Study design
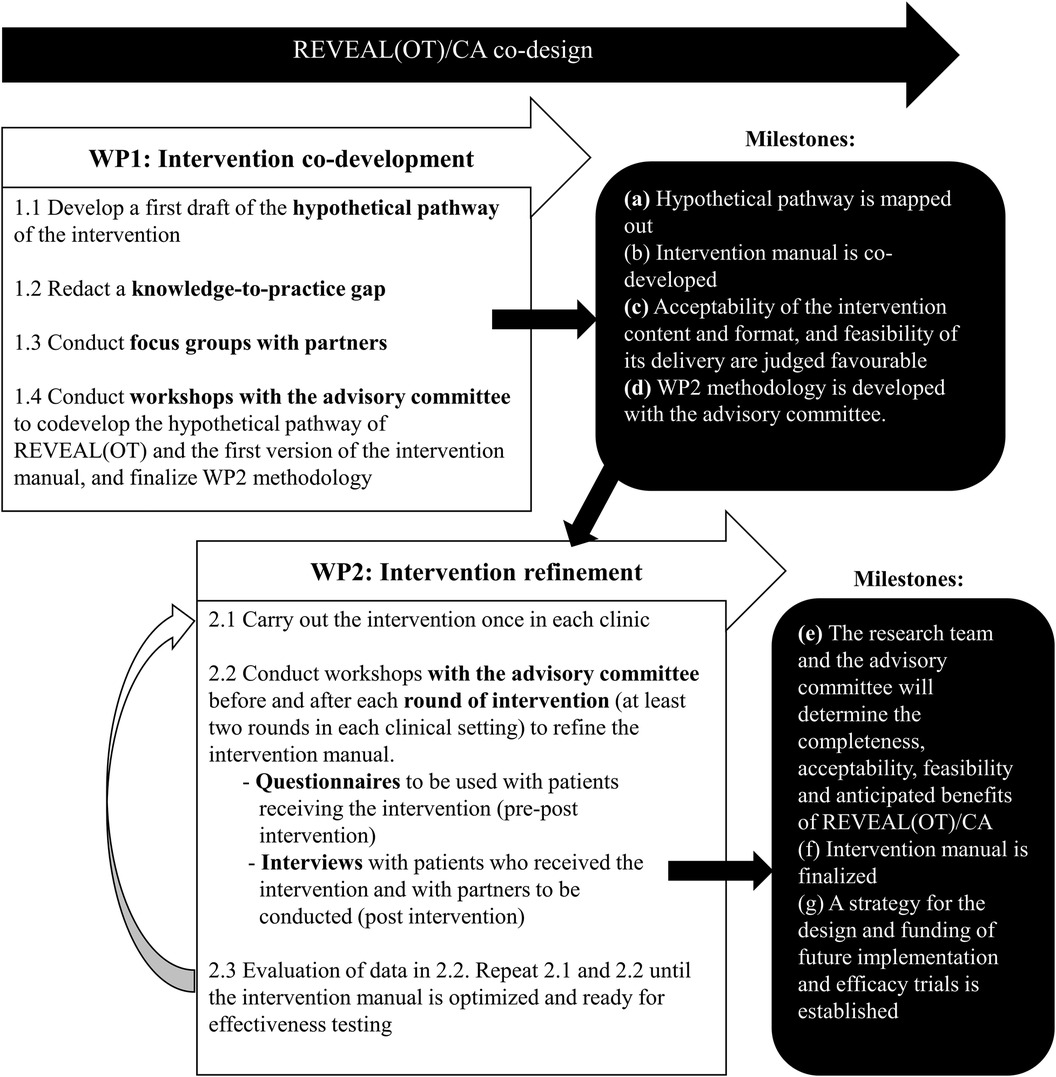
The current project consists of two related work packages (WPs) that aim to codesign a Canadian adaptation of REVEAL(OT) in two Montreal specialized pain clinics. WP1 will provide the scientific basis to define key features of the intervention, co-develop the adaptation of REVEAL(OT)'s manual, and theoritically assess acceptability and feasibility of its content and format in collaboration with partners and the advisory committee (Obj. 1 and 2). This will be achieved by conducting intervention development focus groups (n = 86 partners) and workshops. Partners recruited for WP1 (intervention development) will include patients, clinicians and managers from both pain clinics. In order to refine the intervention, WP2 will consist in delivery of the intervention (at least twice in each of two specialized pain clinics; n ≥ 60 patients) followed by interviews with patients who received the intervention and WP1 partners (or new ones if necessary). This step will thus explore its acceptability, feasibility and mechanisms of action both quantitatively and qualitatively (Obj. 3) in order to achieve an improved version of REVEAL(OT)/CA which will fit the two targeted clinical settings and plan future studies (Obj. 4). A visual model of the two WPs is presented in Figure 1 and the protocol for each WP is described in detail below.
Work package 1: definition of REVEAL(OT)/CA
To begin, we will co-define the key features of the intervention based on the following steps (43, 44, 47–50):
(1.1) develop a first draft of the hypothetical pathway of the intervention using the English translation of the original intervention and reviewing scientific knowledge and clinical guidelines about lifestyle-oriented interventions in CP management,
(1.2) identify knowledge-to-practice gaps taking into consideration organizational practices, policies and procedures of the two targeted clinical settings,
(1.3) conduct intervention development focus groups with partners using the hypothetical pathway and the synthesis of the knowledge-to-practice gap as the focus of discussion, to inform the adaptation of the intervention and to assess the theoretical acceptability of the intervention content and format, and feasibility of its delivery, and
(1.4) conduct workshops with the advisory committee to co-develop the hypothetical pathway of REVEAL(OT), first version of REVEAL(OT)/CA manual (in French and in English), and WP2 methodology.
Participants and recruitment for the intervention development focus groups
Eligible participants will be (a) those living with pain for more than 3 months, speaking French and/or English, and who have received treatment at one of the two participating pain clinics within the past 12 months; and (b) health care providers working with individuals with CP at the participating pain clinics for more than 12 months, and managers officially involved in administrative and/or managerial tasks involving the pain clinics. A purposive maximum variation sampling will ensure that we include the perspectives related to sex and gender (patients, clinicians, and managers), socio-economic level (patients), urban/rural living (patients), care paths (patients) and healthcare disciplines (clinicians). Patients will be recruited via posters at each clinic site and from invitations sent electronically or in person by the health care team. Clinicians and managers will be invited electronically or in person via emails and posters. The institution's social medias will also be used to disseminate the recruitment posters as needed. A member of the research team will contact interested individuals to assess eligibility and obtain written consent.
Data collection
A brief sociodemographic questionnaire will be completed by all participants using Research Electronic Data Capture (REDCap). Patients, clinicians, and managers will be invited to discuss service gaps in CP interventions regarding occupational needs, and comment on the proposed intervention content and format. To do so, in-person and/or online semi-structured focus groups lasting approximately 90 min will be conducted separately for patients (2–3 groups of 6–8 patients/clinic; n ≈ 24 at each site) and clinicians/managers (1–2 groups of 6–8 clinicians from various backgrounds and 2 managers/clinic; n ≈ 18 at each site) to facilitate sharing (51). The interview guide will be informed by Proctor et al.'s conceptual framework and taxonomy of implementation outcomes (52, 53) and will structure questions around the implementation outcomes identified in this framework including exploration of acceptability, adoption, appropriateness, feasibility, fidelity, implementation cost and penetration. These focus groups will be audio-recorded and transcribed verbatim. One of the core advisory committee members will be assigned for taking minutes and synthesize overall group discussions. These documents will also serve as data to design the adapted version of the intervention.
Sample size and feasibility of recruitment
Approximately 20 patients, ten clinicians and two managers will be recruited in each clinic. Final sample size will be guided by the concept of information power (51, 54, 55), meaning that it will depend on the amount of relevant information obtained as we go on with the interviews.
Data analysis
A two-step framework-guided rapid analysis will first be conducted in order to produce rigorous and rapid results to inform ongoing co-development process of the hypothetical pathway and the intervention manual (56–58) followed by a reflexive thematic analysis in order to deepen our understanding of participants point of view about implementation outcomes. Step 1. Audio recordings and/or verbatim transcripts will first be summarized using a structured template based on Proctor et al.'s taxonomy (52, 53). The template summary table will be developed by one researcher tested by a subgroup of researchers with a single transcript, and then, reviewed and modified. A review of the summaries will be conducted by the lead researcher to ensure consistency in the data being recorded and modifications will be done if necessary (56). Step 2. Summaries will subsequently be consolidated into a matrix (MS Excel document) by participant type (patients vs. clinicians and managers) to identify frequently occurring themes about participants' point of view including illustrative quotes. Within each theme of the structure template (lines) sub-themes (columns) will summarize satisfactory aspects of the hypothetical pathway and intervention manual and areas for improvements while allowing comparison across groups (one tab per group) (56, 59–64). Reflexive thematic analysis. After the hypothetical pathway is mapped out and the first draft of the intervention manual is co-developed with the advisory committee, a six-phase approach to reflexive thematic analysis (59, 65) will be performed in order to provide a richer and more detailed account of data obtained from the focus groups (56, 59, 60, 65, 66). These phases will include familiarization with the data by research team members to gain initial insight of the data through sharing perspectives, generating codes using primarily a deductive approach based on Proctor et al.'s taxonomy (52, 53) while allowing for inductive analysis also to make sense of data, searching, reviewing and defining main themes, and producing the final report. To do so, researchers will revisit the research question, notes from the familiarization phase, lists of codes and theme definitions, while making connections with existing research and literature.
Data validation strategies will include participants' input (three participants from different backgrounds will be re-contacted) and deviant cases (cases not fitting conclusions and account for these differences) (67, 68). Divergent perspectives will be considered in order to broaden our results and discuss the strengths and limits of our findings (69).
Milestones
At the end of WP1, (a) the hypothetical pathway describing the components of the intervention and their potential mechanisms of action on the targeted outcomes, as well as the (b) first version of REVEAL(OT)/CA intervention manual will have been co-developed with the advisory committee; (c) acceptability of the intervention content and format, and feasibility of its delivery will be judged favourable by partners and the advisory committee, and (d) WP2 methodology will have been developed with the advisory committee.
Work package 2: refinement of REVEAL(OT)/CA
Based on WP1 results, we will:
(2.1) run the adapted intervention at least twice in each clinical setting to explore its acceptability, tolerability, acceptability, feasibility and effects (quantitatively and qualitatively), and ultimately achieve an improved version of REVEAL(OT)/CA which will fit the two targeted clinical settings and plan future studies,
(2.2) workshops with the advisory committee before and after each round of intervention will be conducted and
(2.3) analyzed to co-develop and improve the intervention manual. This sequence will be repeated until the intervention manual is optimized and ready for effectiveness testing.
This second phase of the study refers to phase Ib of the ORBIT model and will use a mixed methods approach including a fractional factorial design (70–72). By manipulating specific components of an intervention as independent variables (or factor), fractional factorial designs are efficient ways to reach excellent statistical power even with relatively few participants which are used to estimate the effects of each component of the intervention and to assess their interactions (70, 71, 73, 74). The most important components, identified in WP1, will be examined using this fractional factorial design. An iterative process between intervention delivery with data collection, and refinement of the manual will take place until WP2 milestones are achieved.
Participants and recruitment
Patients
Eligible patients will be adults diagnosed with a CP condition who understand spoken and written French and/or English, have access to the Internet and a virtual platform. Individuals with cancer, active suicidal thoughts, substance misuse, severe psychiatric diagnoses such as psychoses, or those currently completing other intensive group or individual interventions (e.g., group psychotherapy) will be excluded. Patients will be referred for the intervention by one of their clinicians at the pain clinic based on their functional deficit in at least one occupational domain (self-care, leisure, productivity; regardless of severity), and this referral could be done at any time point in their treatment path. If interested, they will meet with a research assistant to confirm eligibility through in-person meeting or phone call, and written consent will be obtained.
Clinicians and managers
Clinicians of the participating pain clinics who have had at least one clinical encounter with a patient participating in the program and managers consulted in WP1 will also be eligible. They will be invited electronically or in person by a member of the research team and written consent will be obtained.
Interventional methods
REVEAL(OT)/CA will be run at least twice at each site. The intervention will be co-led at both sites by the lead occupational therapist and one trained occupational therapist that will be recruited from within the hospital setting (or from the community of practice if not available internally) to start building capacity. The latest version of the intervention manual and script protocols or guidelines to support the conduct of each treatment session will be used to enable intervention delivery as planned (75).
Data collection
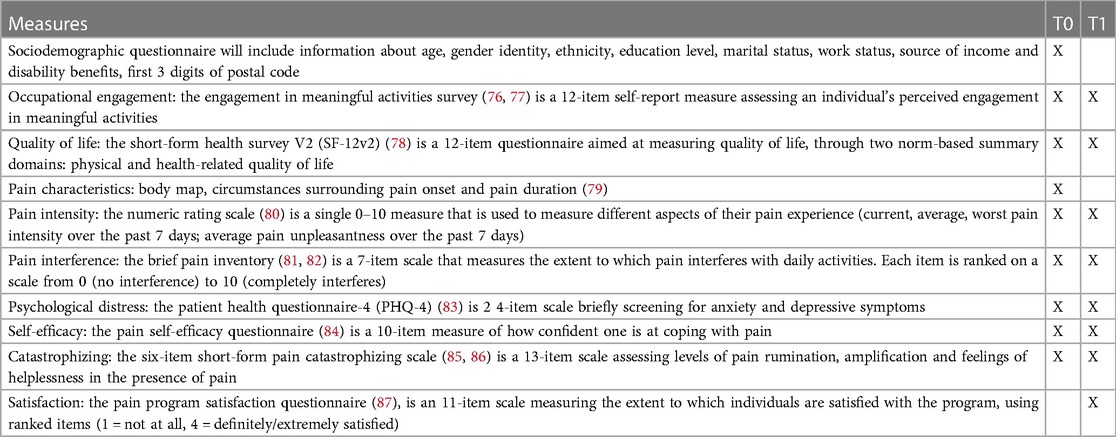
Once determined eligible, recruited patients will provide written consent and be asked to complete a sociodemographic questionnaire followed by a battery of self-report questionnaires within 2 weeks prior to the start of the intervention using the online data capture software (REDCap). These preliminary questionnaires will measure functioning, psychological state, and pain (T0; see Table 1). Final choice of questionnaires will be done in close collaboration with the advisory committee during WP1 workshops. Fidelity to the intervention delivery will be monitored at each session using a pre-established content and behavioral checklist based on the National Institutes of Health Behavior Change Consortium treatment fidelity recommendations and REVEAL(OT)/CA theory and structure (75, 88, 89). This checklist will be completed by clinicians who provide the intervention and research team members who will observe approximately 25% of intervention sessions (or review audio-taped intervention sessions) followed by discussions to correct any deviations in intervention delivery (75, 90–92). Within 2 weeks after the final session, participants (patients) will be invited to complete end-of-treatment measures (T1). Adverse effects (such as unpleasant experiences such as discomfort and cognitive load) (93, 94), as well as process evaluation outcomes (such as information and treatment load) which have been documented in a previous REVEAL(OT) feasibility study (33) will also be collected through a brief survey at the end of each session.
All patients who will have received the intervention, their clinicians, and managers will be invited to participate in semi-structured interviews to document their point of view regarding acceptability of format, content and mode of delivery of the intervention, as well as perceived barriers and benefits, areas for improvement and factors that would influence future implementation of the intervention. These interviews will be conducted online (via Zoom) or in-person and will be audio-recorded and transcribed verbatim. Interview guide will be co-constructed in WP1.
Sample size and feasibility of recruitment
Intervention delivery
Considering that up to 15 patients will be enrolled per group, and considering a 25% attrition rate, it is anticipated that approximately 11 patients would complete the intervention and outcome measures per group for each round of intervention. A sample size of 40 patients receiving the intervention would be sufficient to conduct feasibility assessment in health service intervention (95). There will be a pool of more than 100 patients per clinic per month. Considering that the groups will be run consecutively and not in parallel, we anticipate that we will be able to recruit the 15 patients for each group within a 1-month period prior to each group starting.
Post-intervention interviews
At least four patients, two clinicians and two managers (one form each clinic for each category of participants) will be interviewed. As in WP1, final sample size will be guided by the concept of information power (54, 55).
Quantitative data analyses
Using descriptive statistics (mean, standard deviation, median, minimum and maximum), we will compare sociodemographic data of participants to those of the clinics to examine representativeness of patients exposed to the intervention. Exploratory data analysis will use paired t-tests using pre/post-tests results as all variables are continuous. All results—positive, negative, and inconclusive—will be reported. Analyses will be conducted in R version 4.1.2 or higher.
Qualitative data analysis
Following the same methodology described in WP1, a rapid analysis will again be conducted (51) in order to capture the main preoccupations of our partners (patients who will have received the intervention, their clinicians, and managers consulted in WP1) about what worked well and opportunities for improvement (56, 63). A reflexive thematic analysis will follow in order to deepen our understanding of participants' experience (59, 65). Rapid analysis will now focus on both planned (i.e., changes to be carried out before introducing the intervention) and responsive (i.e., intentional changes resulting from emerging contextual issues during delivery) adaptations of the intervention content and format (42) to achieve an optimal fit between REVEAL(OT) and its new context. Using a similar approach as in WP1, we will use iterative cycles in which workshops with the advisory committee will be conducted to present results after each delivery of REVEL(OT)/CA and pursue its refinement considering quantitative and qualitative findings. In order to capture all aspects of participants experiences with a more nuanced and detailed level, a reflexive thematic analysis will subsequently be performed (59, 60, 65) as explained in WP1. Again, data validation strategies will be used (67, 68) and divergent perspectives will be considered (69). General principles of analysis will include reading through data, memo writing, coding, identifying themes, and interrelating themes. Two cycles of coding will be used (open coding followed by axial coding to examine relationships between codes) (59, 60). WP2 data analysis will lead to a revised version of REVEAL(OT)/CA and an overall strategy for future studies.
Milestones
At the end of this second phase of the study, the research team and the advisory committee will have determined the completeness, acceptability, feasibility and anticipated benefits of REVEAL(OT)/CA, finalized the intervention manual, and established a strategy for the design and funding of a future implementation and efficacy trials.
Discussion
Given the heavy individual, societal and economic impact of CP, and the importance of developing integrative approaches to better manage and treat CP (96), this research project will contribute to fulfilling several gaps identified in the literature by meeting the occupational needs of patients within the Canadian healthcare system. By doing so, it will support rehabilitation as one of the key components of CP management while directly building capacity among occupational therapists through the development of empirically-based effective lifestyle-oriented interventions in chronic pain management. The study methodology uses rigorous intervention development and conceptual models which put an emphasis on partner involvement at all phases of the study to reach an optimal fit between an intervention and its new context (42, 46, 47). Following this iterative process, REVEAL(OT)/CA will be designed to fit two Montreal specialized pain clinics that do not yet include occupational approaches. Not only will this study set the stage for a subsequent randomized controlled trial to test REVEAL(OT)/CA's effectiveness and later implementation, it will also increase visibility for the role and unique contribution of occupational therapy in complementarity with CP management treatments already offered.
Ethics statement
This study was approved (#MP-02-2024-11469) by the research ethics board of the Research Center of the Centre Hospitalier de l'Université de Montréal.
Author contributions
JM: Conceptualization, Methodology, Writing – original draft. SN: Conceptualization, Methodology, Writing – review & editing. JC: Conceptualization, Methodology, Writing – review & editing. SS: Conceptualization, Methodology, Writing – review & editing. JC: Conceptualization, Methodology, Writing – review & editing. SS: Conceptualization, Methodology, Writing – review & editing. ÉL: Conceptualization, Methodology, Writing – review & editing. AB: Conceptualization, Methodology, Writing – review & editing. JP: Conceptualization, Methodology, Writing – review & editing. ML: Conceptualization, Methodology, Writing – review & editing. MP: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research project was supported by funding from the Canadian Institutes for Health Research (no 486429). MP is a Junior 2 research scholar from the Fonds de recherche du Québec en santé.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. (2019) 160(1):19–27. doi: 10.1097/j.pain.0000000000001384
2. Campbell F, Hudspith M, Anderson M. Chronic pain in Canada: laying a foundation for action. In: Health Canada. (2019). p. 1–49. ISBN: 978-0-660-31747-2
3. Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73(6):968. doi: 10.1136/annrheumdis-2013-204428
4. Campbell F, Hudspith M, Choinière M, El-Gabalawy H, Laliberté J, Sangster M, et al. Working together to better understand. Prevent, and manage chronic pain: what we heard. Health Canada. (2020). p. 1–75. ISBN: 978-0-660-36395-0
5. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. (2016) 9:457–67. doi: 10.2147/JPR.S105892
6. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
7. Ervasti J, Vahtera J, Pentti J, Oksanen T, Ahola K, Kivekäs T, et al. The role of psychiatric, cardiometabolic, and musculoskeletal comorbidity in the recurrence of depression-related work disability. Depress Anxiety. (2014) 31(9):796–803. doi: 10.1002/da.22286
8. Keponen R, Kielhofner G. Occupation and meaning in the lives of women with chronic pain. Scand J Occup Ther. (2006) 13(4):211–20. doi: 10.1080/11038120600672975
9. Choinière M, Dion D, Peng P, Banner R, Barton PM, Boulanger A, et al. The Canadian STOP-PAIN project—part 1: who are the patients on the waitlists of multidisciplinary pain treatment facilities? Can J Anaesth. (2010) 57(6):539–48. doi: 10.1007/s12630-010-9305-5
10. Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. (1998) 280(2):147–51. doi: 10.1001/jama.280.2.147
11. Jonsdottir T, Aspelund T, Jonsdottir H, Gunnarsdottir S. The relationship between chronic pain pattern, interference with life and health-related quality of life in a nationwide community sample. Pain Manag Nurs. (2014) 15(3):641–51. doi: 10.1016/j.pmn.2013.07.005
12. Langley P, Müller-Schwefe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G. The societal impact of pain in the European union: health-related quality of life and healthcare resource utilization. J Med Econ. (2010) 13(3):571–81. doi: 10.3111/13696998.2010.516709
13. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. (2006) 10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009
14. Resnik DB, Rehm M. The undertreatment of pain: scientific, clinical, cultural, and philosophical factors. Med Health Care Philos. (2001) 4(3):277–88. doi: 10.1023/A:1012057403159
15. Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. (2011) 377(9784):2226–35. doi: 10.1016/S0140-6736(11)60402-9
16. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. (2014) 69(2):119–30. doi: 10.1037/a0035514
17. Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain. (1992) 49(2):221–30. doi: 10.1016/0304-3959(92)90145-2
18. Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). (2008) 47(5):670–8. doi: 10.1093/rheumatology/ken021
19. Pagé MG, Romero Escobar EM, Ware MA, Choinière M. Predicting treatment outcomes of pain patients attending tertiary multidisciplinary pain treatment centers: a pain trajectory approach. Can J Pain. (2017) 1(1):61–74. doi: 10.1080/24740527.2017.1325715
20. Campbell F, Hudspith M, Anderson M. An action plan for pain in Canada. Health Canada. (2021). p. 1–20. ISBN: 978-0-660-38498-6
21. Fisher GS, Emerson L, Firpo C, Ptak J, Wonn J, Bartolacci G. Chronic pain and occupation: an exploration of the lived experience. Am J Occup Ther. (2007) 61(3):290–302. doi: 10.5014/ajot.61.3.290
22. Prefontaine K, Rochette A. A literature review on chronic pain: the daily overcoming of a complex problem. Br J Occup Ther. (2013) 76(6):280–6. doi: 10.4276/030802213X13706169932905
23. Persson E, Lexell J, Rivano-Fischer M, Eklund M. Everyday occupational problems perceived by participants in a pain rehabilitation programme. Scand J Occup Ther. (2013) 20(4):306–14. doi: 10.3109/11038128.2013.793739
24. Nielsen SS, Skou ST, Larsen AE, Søndergaard J, Christensen JR. Associations of health-related quality of life with sociodemographic characteristics, health, pain, and lifestyle factors, and motivation for changing lifestyle in adults living with chronic pain: a cross-sectional exploratory study. Scand J Pain. (2022) 22(1):142–53. doi: 10.1515/sjpain-2021-0062
25. Goodall J, Brown T. Patients’ experiences of occupational therapy within a multidisciplinary pain management programme: a qualitative study. Br J Pain. (2022) 16(3):290–302. doi: 10.1177/20494637211054697
26. Nijs J, D’Hondt E, Clarys P, Deliens T, Polli A, Malfliet A, et al. Lifestyle and chronic pain across the lifespan: an inconvenient truth? PM R. (2020) 12(4):410–9. doi: 10.1002/pmrj.12244
27. IASP curriculum outline on pain for occupational therapy. International Association for the Study of Pain (IASP). Available at: https://www.iasp-pain.org/education/curricula/iasp-curriculum-outline-on-pain-for-occupational-therapy/ (Cité 14 Mars 2023)
28. Lagueux É, Dépelteau A, Masse J. Occupational therapy’s unique contribution to chronic pain management: a scoping review. Pain Res Manag. (2018) 2018:5378451. doi: 10.1155/2018/5378451
29. Hill W. The role of occupational therapy in pain management. Anaesth Intensive Care Med. (2016) 17(9):451–3. doi: 10.1016/j.mpaic.2016.06.008
30. Lagueux É, Masse J, Pagé R, Marin B, Tousignant-Laflamme Y. Management of chronic pain by occupational therapist: a description of practice profile. Can J Occup Ther. (2023):84174231162709. doi: 10.1177/00084174231162709
31. Locas V, Préfontaine C, Veillette N, Vachon B. Integration of occupational therapists into family medicine groups: physicians’ perspectives. Br J Occup Ther. (2020) 83(7):458–68. doi: 10.1177/0308022619883481
32. Lagueux É, Masse J, Levasseur M, Pagé R, Dépelteau A, Lévesque MH, et al. Pilot study of French-Canadian lifestyle redesign® for chronic pain management. OTJR (Thorofare N J). (2021) 41(2):80–9. doi: 10.1177/1539449220982908
33. Nielsen SS, Christensen JR, Søndergaard J, Mogensen VO, Enemark Larsen A, Skou ST, et al. Feasibility assessment of an occupational therapy lifestyle intervention added to multidisciplinary chronic pain treatment at a Danish pain centre: a qualitative evaluation from the perspectives of patients and clinicians. Int J Qual Stud Health Well-Being. (2021) 16(1):1949900. doi: 10.1080/17482631.2021.1949900
34. Simon A U, Collins CER. Lifestyle redesign® for chronic pain management: a retrospective clinical efficacy study. Am J Occup Ther. (2017) 71(4):7104190040p1–7. doi: 10.5014/ajot.2017.025502
35. Nielsen SS, Skou ST, Larsen AE, Polianskis R, Pawlak WZ, Vægter HB, et al. Occupational therapy lifestyle intervention added to multidisciplinary treatment for adults living with chronic pain: a feasibility study. BMJ Open. (2022) 12(9):e060920. doi: 10.1136/bmjopen-2022-060920
36. Nielsen SS, Christensen JR, Søndergaard J, Surrow S, Enemark Larsen A, Skou ST, et al. “It has changed my picture of myself”: how did females living with chronic pain perceive the impact of the standard pain rehabilitation, including the occupational therapy lifestyle intervention REVEAL(OT)? Disabil Rehabil. (2023) 0(0):1–12. doi: 10.1080/09638288.2023.2230127
37. Nielsen SS, Skou ST, Larsen AE, Bricca A, Søndergaard J, Christensen JR. The effect of occupational engagement on lifestyle in adults living with chronic pain: a systematic review and meta-analysis. Occup Ther Int. (2022) 2022:7082159. doi: 10.1155/2022/7082159
38. van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology—where do lifestyle factors fit in? Br J Pain. (2013) 7(4):209–17. doi: 10.1177/2049463713493264
39. Eklund M, Erlandsson LK. Women’s perceptions of everyday occupations: outcomes of the redesigning daily occupations (ReDO) programme. Scand J Occup Ther. (2014) 21(5):359–67. doi: 10.3109/11038128.2014.922611
40. Eklund M, Wästberg BA, Erlandsson LK. Work outcomes and their predictors in the redesigning daily occupations (ReDO) rehabilitation programme for women with stress-related disorders. Aust Occup Ther J. (2013) 60(2):85–92. doi: 10.1111/1440-1630.12019
41. Pyatak EA, Carandang K, Davis S. Developing a manualized occupational therapy diabetes management intervention: resilient, empowered, active living with diabetes. OTJR (Thorofare N J). (2015) 35(3):187–94. doi: 10.1177/1539449215584310
42. Moore G, Campbell M, Copeland L, Craig P, Movsisyan A, Hoddinott P, et al. Adapting interventions to new contexts-the ADAPT guidance. Br Med J. (2021) 374:n1679. doi: 10.1136/bmj.n1679
43. Powell LH, Kaufmann PG, Freedland KE. Behavioral treatment development. In: Powell LH, Freedland KE, Kaufmann PG, éditeurs. Behavioral clinical trials for chronic diseases: Scientific foundations. Cham: Springer International Publishing (2021). p. 27–68. doi: 10.1007/978-3-030-39330-4_3 (Cité 17 Mai 2023)
44. Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, et al. From ideas to efficacy: the ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. (2015) 34(10):971–82. doi: 10.1037/hea0000161
45. Bacon SL, Campbell TS, Lavoie KL. Rethinking how to expand the evidence base for health behavior change in cardiovascular disease prevention. J Am Coll Cardiol. (2020) 75(20):2619–22. doi: 10.1016/j.jacc.2020.03.055
46. Bacon SL, Lavoie KL, Ninot G, Czajkowski S, Freedland KE, Michie S, et al. An international perspective on improving the quality and potential of behavioral clinical trials. Curr Cardiovasc Risk Rep. (2014) 9(1):427. doi: 10.1007/s12170-014-0427-0
47. Chambers D, Glasgow R, Stange K. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implementation science: IS. (2013) 8. Available at: https://pubmed.ncbi.nlm.nih.gov/24088228/ (Cité 20 Déc 2022).
48. Shojania KG, McDonald KM, Wachter RM, Owens DK, éditeurs. Closing the quality gap: A critical analysis of quality improvement strategies (vol. 1: Series overview and methodology). Rockville, MD: Agency for Healthcare Research and Quality (US) (2004). p. 1–48. (AHRQ Technical Reviews). Available at: http://www.ncbi.nlm.nih.gov/books/NBK43908/ (Cité 17 Mai 2023)
49. Golden SH, Hager D, Gould LJ, Mathioudakis N, Pronovost PJ. A gap analysis needs assessment tool to drive a care delivery and research agenda for integration of care and sharing of best practices across a health system. Jt Comm J Qual Patient Saf. (2017) 43(1):18–28. doi: 10.1016/j.jcjq.2016.10.004
50. Nyanchoka L, Tudur-Smith C, Thu VN, Iversen V, Tricco AC, Porcher R. A scoping review describes methods used to identify, prioritize and display gaps in health research. J Clin Epidemiol. (2019) 109:99–110. doi: 10.1016/j.jclinepi.2019.01.005
52. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38(2):65–76. doi: 10.1007/s10488-010-0319-7
53. Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. (2009) 36(1):24–34. doi: 10.1007/s10488-008-0197-4
54. Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. (2016) 26(13):1753–60. doi: 10.1177/1049732315617444
55. Camic PM. Qualitative research in psychology: Expanding perspectives in methodology and design. 2e édition Washington, DC, US: American Psychological Association (2021). p. 323. xiii.
56. Gale RC, Wu J, Erhardt T, Bounthavong M, Reardon CM, Damschroder LJ, et al. Comparison of rapid vs. in-depth qualitative analytic methods from a process evaluation of academic detailing in the veterans health administration. Implement Sci. (2019) 14(1):11. doi: 10.1186/s13012-019-0853-y
57. Nevedal AL, Reardon CM, Opra Widerquist MA, Jackson GL, Cutrona SL, White BS, et al. Rapid vs. traditional qualitative analysis using the consolidated framework for implementation research (CFIR). Implement Sci. (2021) 16(1):67. doi: 10.1186/s13012-021-01111-5
58. Ramanadhan S, Revette AC, Lee RM, Aveling EL. Pragmatic approaches to analyzing qualitative data for implementation science: an introduction. Implement Sci Commun. (2021) 2(1):70. doi: 10.1186/s43058-021-00174-1
59. Braun V. Thematic analysis. In: Liamputtong P, editor. Handbook of research methods in health social sciences. Singapore: Springer Singapore (2019). p. 843–60.
60. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: implications for conducting a qualitative descriptive study. Nurs Health Sci. (2013) 15(3):398–405. doi: 10.1111/nhs.12048
61. Solomon P, Nixon S, Bond V, Cameron C, Gervais N. Two approaches to longitudinal qualitative analyses in rehabilitation and disability research. Disabil Rehabil. (2020) 42(24):3566–72. doi: 10.1080/09638288.2019.1602850
62. Raskind IG, Shelton RC, Comeau DL, Cooper HLF, Griffith DM, Kegler MC. A review of qualitative data analysis practices in health education and health behavior research. Health Educ Behav. (2019) 46(1):32–9. doi: 10.1177/1090198118795019
63. Johnson JL, Adkins D, Chauvin S. A review of the quality indicators of rigor in qualitative research. Am J Pharm Educ Janv. (2020) 84(1):7120. doi: 10.5688/ajpe7120
64. Bonner C, Tuckerman J, Kaufman J, Costa D, Durrheim DN, Trevena L, et al. Comparing inductive and deductive analysis techniques to understand health service implementation problems: a case study of childhood vaccination barriers. Implement Sci Commun. (2021) 2(1):100. doi: 10.1186/s43058-021-00202-0
65. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. (2006) 3(2):77–101. doi: 10.1191/1478088706qp063oa
66. Coates WC, Jordan J, Clarke SO. A practical guide for conducting qualitative research in medical education: part 2—coding and thematic analysis. AEM Educ Train. (2021) 5(4):e10645. doi: 10.1002/aet2.10645
67. Becker H. Logic. In: Tricks of the trade: How to think about your research while you’re doing it. Chigago: University of Chicago Press (1998). p. 146.
68. Creswell JW, Creswell JD. Research design: Qualitative, quantitative, and mixed methods approaches. 6th edition. Los Angeles: SAGE (2023). 291. xxvii.
69. Hanson A. Negative case analysis. In: Matthes J, Davis CS, Potter RF, editors. The international encyclopedia of communication research methods. (2017). p. 165. doi: 10.1002/9781118901731.iecrm0165
70. Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. (2014) 47(4):498–504. doi: 10.1016/j.amepre.2014.06.021
71. Dziak JJ, Nahum-Shani I, Collins LM. Multilevel factorial experiments for developing behavioral interventions: power, sample size, and resource considerations. Psychol Methods. (2012) 17(2):153–75. doi: 10.1037/a0026972
72. Baker TB, Smith SS, Bolt DM, Loh WY, Mermelstein R, Fiore MC, et al. Implementing clinical research using factorial designs: a primer. Behav Ther. (2017) 48(4):567–80. doi: 10.1016/j.beth.2016.12.005
73. Nair V, Strecher V, Fagerlin A, Ubel P, Resnicow K, Murphy S, et al. Screening experiments and the use of fractional factorial designs in behavioral intervention research. Am J Public Health. (2008) 98(8):1354–9. doi: 10.2105/AJPH.2007.127563
74. Chakraborty B, Collins LM, Strecher VJ, Murphy SA. Developing multicomponent interventions using fractional factorial designs. Stat Med. (2009) 28(21):2687–708. doi: 10.1002/sim.3643
75. Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. (2004) 23(5):443–51. doi: 10.1037/0278-6133.23.5.443
76. Eakman AM, Carlson M, Clark F. Factor structure, reliability and convergent validity of the engagement in meaningful activities survey for older adults. OTJR (Thorofare N J). (2010) 30(3):111–21. doi: 10.3928/15394492-20090518-01
77. Eakman AM, Carlson ME, Clark FA. The meaningful activity participation assessment: a measure of engagement in personally valued activities. Int J Aging Hum Dev. (2010) 70(4):299–317. doi: 10.2190/AG.70.4.b
78. Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. Mars. (1996) 34(3):220–33. doi: 10.1097/00005650-199603000-00003
79. Choinière M, Ware MA, Pagé MG, Lacasse A, Lanctôt H, Beaudet N, et al. Development and implementation of a registry of patients attending multidisciplinary pain treatment clinics: the Quebec pain registry. Pain Res Manag. (2017) 2017:8123812. doi: 10.1155/2017/8123812
80. Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: iMMPACT recommendations. Pain. (2005) 113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012
81. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. (1994) 23(2):129–38. 8080219.8080219
82. Poundja J, Fikretoglu D, Guay S, Brunet A. Validation of the French version of the brief pain inventory in Canadian veterans suffering from traumatic stress. J Pain Symptom Manage. (2007) 33(6):720–6. doi: 10.1016/j.jpainsymman.2006.09.031
83. Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. (2009) 50(6):613–21. doi: 10.1176/appi.psy.50.6.613
84. Katz J, Fashler SR, Wicks C, Pagé MG, Roosen KM, Kleiman V, et al. Sensitivity to pain traumatization scale: development, validation, and preliminary findings. J Pain Res. (2017) 10:1297–316. doi: 10.2147/JPR.S134133
85. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7(4):524. doi: 10.1037/1040-3590.7.4.524
86. McWilliams LA, Kowal J, Wilson KG. Development and evaluation of short forms of the pain catastrophizing scale and the pain self-efficacy questionnaire. Eur J Pain. (2015) 19(9):1342–9. doi: 10.1002/ejp.665
87. Li YC, Hapidou EG. Patient satisfaction with chronic pain management: patient perspectives of improvement. J Patient Exp. (2021) 8:23743735211007834. doi: 10.1177/23743735211007834
88. Anis L, Benzies KM, Ewashen C, Hart MJ, Letourneau N. Fidelity assessment checklist development for community nursing research in early childhood. Front Public Health. (2021) 9:1–14. doi: 10.3389/fpubh.2021.582950
89. Song MK, Happ MB, Sandelowski M. Development of a tool to assess fidelity to a psycho-educational intervention. J Adv Nurs. (2010) 66(3):673–82. doi: 10.1111/j.1365-2648.2009.05216.x
90. McGuire R, Duncan K, Pozehl B. Incorporating intervention fidelity components into RCT promoting exercise adherence in heart failure patients. Res Nurs Health. (2019) 42(4):306–16. doi: 10.1002/nur.21949
91. Wickersham K, Colbert A, Caruthers D, Tamres L, Martino A, Erlen JA. Assessing fidelity to an intervention in a randomized controlled trial to improve medication adherence. Nurs Res. (2011) 60(4):264–9. doi: 10.1097/NNR.0b013e318221b6e6
92. Kepper MM, Walsh-Bailey C, Brownson RC, Kwan BM, Morrato EH, Garbutt J, et al. Development of a health information technology tool for behavior change to address obesity and prevent chronic disease among adolescents: designing for dissemination and sustainment using the ORBIT model. Front Digit Health. (2021) 3. doi: 10.3389/fdgth.2021.648777
93. Amalberti R, Benhamou D, Auroy Y, Degos L. Adverse events in medicine: easy to count, complicated to understand, and complex to prevent. J Biomed Inform. (2011) 44(3):390–4. doi: 10.1016/j.jbi.2009.06.004
94. Adams-Phipps J, Toomey D, Więcek W, Schmit V, Wilkinson J, Scholl K, et al. A systematic review of human challenge trials, designs, and safety. Clin Infect Dis. (2023) 76(4):609–19. doi: 10.1093/cid/ciac820
95. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. (2004) 10(2):307–12. doi: 10.1111/j..2002.384.doc.x
Keywords: chronic pain, occupational therapy, REVEAL(OT), participatory research, implementation science
Citation: Masse J, Nielsen SS, Christensen JR, Skou ST, Côté J, Saunders S, Lagueux É, Boulanger A, Perez-Martinez J, Lussier M and Pagé MG (2023) Co-designing a Canadian adaptation of a lifestyle-oriented intervention aimed to improve daily functioning of individuals living with chronic pain: a multi-method study protocol of REVEAL(OT) Canada. Front. Rehabil. Sci. 4:1281680. doi: 10.3389/fresc.2023.1281680
Received: 22 August 2023; Accepted: 6 November 2023;
Published: 24 November 2023.
Edited by:
Feng Yang, Georgia State University, United StatesReviewed by:
Dominique Van De Velde, Ghent University, BelgiumChungyi Chiu, University of Illinois at Urbana-Champaign, United States
© 2023 Masse, Nielsen, Christensen, Skou, Côté, Saunders, Lagueux, Boulanger, Perez-Martinez, Lussier and Pagé. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. G. Pagé gabrielle.page@umontreal.ca
 J. Masse
J. Masse S. S. Nielsen3,4
S. S. Nielsen3,4  J. R. Christensen
J. R. Christensen M. G. Pagé
M. G. Pagé