Abstract
Predators in food webs are valuable sentinel species for zoonotic and multi-host pathogens such as Toxoplasma gondii. This protozoan parasite is ubiquitous in warm-blooded vertebrates, and can have serious adverse effects in immunocompromised hosts and foetuses. In northern ecosystems, T. gondii is disproportionately prevalent in Inuit people and wildlife, in part due to multiple routes of transmission. We combined data on T. gondii infection in foxes from Nunavik (northern Québec, Canada) with stable isotope data tracking trophic relationships between foxes and several of their main prey species. Red (Vulpes vulpes) and Arctic fox (Vulpes lagopus) carcasses were collected by local trappers from 2015 to 2019. We used magnetic capture PCR to detect DNA of T. gondii in heart and brain tissues, and enzyme-linked immunosorbent assay to detect antibodies in blood. By linking infection status with diet composition, we showed that infected foxes had a higher probability of consuming aquatic prey and migratory geese, suggesting that these may be important sources of T. gondii transmission in the Arctic. This use of stable isotopes to reveal parasite transmission pathways can be applied more broadly to other foodborne pathogens, and provides evidence to assess and mitigate potential human and animal health risks associated with T. gondii in northern ecosystems.
Similar content being viewed by others
Introduction
The protozoan Toxoplasma gondii is a ubiquitous, zoonotic parasite of public health significance. In humans, T. gondii can cause life-threatening infections, especially in immunocompromised hosts and foetuses1,2. Exposure to the parasite in some Inuit communities in Nunavik, northern Québec, Canada, is much higher than in other parts of North America (43% seroprevalence compared to 10–15%)3. Inuit are thought to be primarily exposed through consumption of terrestrial and aquatic Arctic wildlife, often prepared in ways that would not inactivate the parasite3,4. Still, transmission of T. gondii in terrestrial Arctic ecosystems is complex, potentially involving food, water and vertical routes, and food-borne source attribution is often unclear. Northern transmission of T. gondii could involve food or water contaminated with oocysts3,5; oocysts of T. gondii have been observed in aquatic filter feeders (i.e., fish, oyster, clam, snail)6,7. However, true food-borne transmission from ingesting tissue cysts of the parasite in infected intermediate hosts (versus exposure to oocysts) is thought to be significant in the Arctic, as wild and domestic felids, the only known source of oocysts in the environment, are mostly absent in tundra and high Arctic ecosystems8. Therefore, northern hosts may be exposed through the consumption of cysts of T. gondii in the tissues of chronically infected migratory prey, such as caribou (Rangifer tarandus), Arctic-nesting geese (e.g. Branta canadensis, Anser caerulescens) or marine mammals5,9,10,11 (Fig. 1).

(Reprinted and modified with permission from Springer Nature: Springer, Toxoplasmosis in Northern Regions, Bouchard et al.12).
Potential routes of transmission of the zoonotic parasite Toxoplasma gondii in the North, with focus on free-ranging wildlife hosts and the shared environment
Given their generalist predator and/or scavenger foraging behaviors across multiple trophic levels, and their close association with human activities, foxes (Vulpes spp.) are potentially good sentinel species to investigate T. gondii transmission through food webs. Indeed, exposure to T. gondii (i.e., seroprevalence) in foxes tracks closely with that of people in many regions of the Canadian North13. However, documenting trophic routes through which people and foxes in remote regions are infected with T. gondii remains challenging. Stable isotope analysis, an indirect diet reconstruction approach, has been used to study the diet of terrestrial species, including foxes14,15,16,17,18. Carbon (δ13C) and nitrogen (δ15N) isotopic values of consumer tissues reflect dietary composition, after accounting for trophic enrichment against heavier isotopes and assimilation19,20. Carbon isotopic ratios distinguish between terrestrial and marine food sources, while nitrogen isotopic ratios are a relative index of trophic position of individuals or species21,22. Different body parts provide dietary information that is integrated over different time scales (e.g., days to months), due to the turnover rate of isotopes19,20,23. For example, isotopic ratios of blood cells and muscle tissue provide insights on the food habits of a consumer over the past few weeks, while hair reflects the diet of the animal during the entire growth period of the hair since the last molt23.
In this study, we combined data on stable isotope analysis and pathogen infection status to determine possible routes of transmission of T. gondii in a highly affected region of the Canadian Arctic3. It has been suggested that T. gondii may enter the terrestrial Arctic ecosystem of northern Norway via migratory birds24; an estimated 7% of barnacle geese (Branta leucopsis) on Svalbard, where no wild or domestic felids are present, are exposed to the parasite5. Previous work on migratory geese and marine mammals in Nunavik found a seroprevalence of 11% in migratory geese (primarily Branta canadensis), and 20% in ringed seals (Pusa hispida)25, and recent findings in the 2017 Nunavik Inuit Health Survey reported higher seroprevalence for T. gondii in Inuit with consumption of marine mammals (especially seal), fish, and geese3. We therefore hypothesize that migratory geese and aquatic hosts consumed by foxes may be primarily responsible for transporting T. gondii to the Arctic and disseminating the parasite within northern ecosystems. Given the zoonotic risk posed by T. gondii in Inuit communities, documenting its prevalence and transmission routes in northern wildlife is crucial to understanding and addressing the risk of infection in vulnerable animal and human populations. More broadly, we demonstrate that linking epidemiological and ecological approaches provides a powerful method to unravel transmission of T. gondii and other food-borne pathogens with complex transmission routes.
Methods
Study area
The climate in Nunavik, northern Québec (Canada), is heavily influenced by two large water bodies, Hudson Bay and Ungava Bay (Fig. 2), with ice covering the sea from November to July. The northern part of Nunavik has an Arctic climate, continuous permafrost, tundra vegetation, and an annual average temperature of − 7.5 °C and precipitation of 300 mm. The southern, inland part of Nunavik has a Subarctic climate, discontinuous permafrost, taiga vegetation and an average annual temperature of − 2.5 °C and precipitation of 700 mm26. Potential terrestrial food sources (through predation and scavenging) for Arctic and red foxes include lemmings (Lemmus spp.), voles (Microtus spp.), Arctic hares (Lepus arcticus), Arctic ground squirrels (Urocitellus parryii), migratory woodland caribou (Rangifer tarandus caribou), muskoxen (Ovibos moschatus), and Arctic nesting geese (e.g. Branta canadensis, Anser caerulescens)27,28. Both fox species rely heavily on lemmings and voles29. Foxes may also scavenge carcasses of marine wildlife30,31, including beluga (Delphinapterus leucas), walrus (Odobenus rosmarus), seals (Phoca hispida, Erignathus barbatus), and fish, as natural mortalities or harvested by human residents32.

(Reprinted and modified with permission from Makivvik Corporation (https://www.makivvik.ca/wp-content/uploads/2013/02/nunavik1.gif).
Communities (black circles) and surrounding areas where foxes (Vulpes spp.) were collected during winter 2015–2019 by trappers from Nunavik, Canada
Fox sampling
This study was part of a larger research project on T. gondii in foxes across northern Canada13. Fox carcasses were harvested by local trappers during regular, licensed fur-trapping activities during winters of 2015–2019 (between December and February) in two regions of Nunavik: eastern Hudson Bay (Arctic foxes = 23, red foxes = 51) and southwestern Ungava Bay (Arctic foxes = 19, red foxes = 146) (Fig. 2). Following consultation, interested communities self-elected for participation, and local coordinators were recruited to ensure that carcasses were stored at − 20 °C until shipped for necropsy.
Frozen carcasses were sent for necropsies at the Western College of Veterinary Medicine in Saskatoon (SK), the Faculté de Médecine Vétérinaire in Saint-Hyacinthe (QC), and the Nunavik Research Centre in Kuujjuaq (QC). Acknowledging that trappers may have superficially cross-contaminated fox carcasses while skinning, we sampled deep muscle tissue and internal organs using clean tools between carcasses. From each fox, we collected whole hearts, brains, thigh muscle, and hair (extracted by tweezers from root to tip from residual hair patches left on the right hind paw by trappers). Brain and heart tissues are predilection sites for T. gondii in animals33,34,35. We recorded harvest location, species, and sex for all individuals. In accordance with the Canadian Council on Animal Care guidelines, this research was exempt from Animal Research Ethic Board review because all samples were collected from animals legally harvested for non-research purposes in accordance with relevant guidelines and regulations.
Serological analysis
As validated in Sharma et al.36 and described in Bouchard et al.13, we detected antibodies to T. gondii in fluid from thawed heart tissue (diluted 1:2) using the commercially available ID Screen® Toxoplasmosis Indirect Multi-species Enzyme-linked immunosorbent assay (ELISA) (IDvet, Grabels, France). Samples with an S/P % less than or equal to 40% were considered negative. Samples greater than or equal to 70% were considered positive. If the S/P % was between 40 and 70%, the test result was considered doubtful, and testing was repeated. The ELISA relative sensitivity and specificity using magnetic capture PCR as a reference test was 94% and 100%, respectively37.
Molecular analyses
As validated by Opsteegh et al.38 and described in Bouchard et al.13, sequence specific DNA of T. gondii was extracted by magnetic capture from whole heart and brain tissues combined for each fox followed by real-time PCR using the Tox 9F (5′-aggagagata tcaggactgtag-3′) and Tox 11R (5′-gcgtcgtctc gtctagatcg-3′) primers, for the detection of the 188 bp T. gondii sequence within the 529 repeat-element. Each run on magnetic capture included two spiked beef samples (positive controls) and one beef sample without spiking (negative control). A reaction was considered positive if (1) the Cq value was less than or equal to 35, (2) the two positive extraction controls were positive, and (3) the negative and two no-template controls were negative. Reactions with Cq values between 35 and 40 were considered positive if a 188 bp band was identified on gel electrophoresis.
Collection of food sources
Potential major food sources of foxes were collected from a concurrent study in Nunavik22. Briefly, hair and muscle samples from prey were collected in 2016–2017 and 2017–2018 in the same geographic regions as foxes. We selected three dietary endpoints of red and Arctic fox diets29: hair from Ungava collared lemmings (Dicrostonyx hudsonius), breast muscle from Canada geese collected during their southern migration (Aug-Sept) (Branta canadensis), and ventral muscle of fish, both anadromous Arctic char (Salvelinus alpinus) and freshwater lake trout (Salvelinus namaycush). Geese, Arctic char, and lake trout were previously found to be infected with T. gondii in the region25,39, making them plausible sources of exposure. For logistical reasons, we did not attempt to fully describe all potential dietary sources of foxes, but rather to focus on distinct isotopic endpoints that represent different sources of T. gondii introduction (resident terrestrial vs migratory geese vs aquatic).
Stable isotope analysis
We collected hair and muscles from foxes trapped in winter. Since foxes molt twice per year14,40, with the autumn molt starting in September, stable isotope signatures from winter fur in our study represented diet composition of foxes from the preceding fall (September to December). Isotopic ratios of muscle tissues from foxes in our study represented their diet composition during winter (December to February)17. Variations in lipid concentration can significantly influence δ13C measurements41; therefore, we cleaned fox and lemming hair with distilled water, soaked 3 times for 10 min in a 2:1 chloroform:methanol solution to remove lipids, then rinsed hair again in distilled water before drying at room temperature for 48 h. We cut multiple, complete hairs in 1 mm segments into tin cups to achieve a total weight of 1 mg from each fox or lemming. We lyophilised muscle tissues (foxes and food sources) for 36 h and ground them in a ball mill. To remove lipids, we added a 2:1 chloroform:methanol solution to each muscle sample, mixed with a tube stirrer, and centrifuged for 8 min at 10,000 RPM. The supernatant was removed, and we repeated the procedure until the supernatant was clear. The samples were left to dry for 24 h, then 1 mg was weighed into tin cups42.
We analysed muscle and hair samples from foxes and prey for nitrogen and carbon at the Laboratoire d’Océanographie of Laval University, Québec (Canada). Isotopic analyses were performed by continuous-flow isotope ratio mass spectrometer (Thermo Electron Delta Advantage) using an ECS 4010 Elemental Analyzer/Zero Blank Autosampler (Costech Analytical Technologies). Stable isotope ratios were expressed in δ notation as parts per thousand (‰) deviation from V-Pee Dee Belemnite (carbon) and AIR (nitrogen) international standards. Measurement precision was ± 0.2‰ for δ13C and ± 0.1‰ for δ15N.
Statistical analysis
Serological and molecular test agreement
Proportion of positive results was compared between ELISA and MC-qPCR, using McNemar’s Chi-square tests for paired data. The kappa coefficient (k) was used to determine the level of agreement between the two tests. Analyses were performed using IBM SPSS (ver. 26; Armonk, New York, USA).
Prevalence and risk factors
Seroprevalence, tissue prevalence and their 95% confidence intervals (CI) were calculated from the proportion of positive results using EpiTools epidemiological calculators43. We used linear regression to test for the effects of sex, species, region, and status of infection on stable isotope values of fox hair and muscles. We used a logistic regression with package lme4 v.1.1–2644 in R v.3.6.345 to evaluate the effect of species, sex, region, terrestrial, aquatic, and migratory geese dietary sources on T. gondii prevalence (a fox was considered positive if it was positive on either serology or molecular testing). Foxes with missing data were not included in the regression. We tested the relevance of possible interaction terms by comparing models with Akaike information criterion (AIC), where models with ΔAIC < 2 were considered equally plausible. Models were tested against the null model to see if there was a significant amelioration (Table 1). The models considered were based on hypotheses of risk factors influencing T. gondii prevalence in foxes (i.e., region, diet, sex and species)13. We did not include year as a fixed effect in our model as it was dependent on region. We did include year as a random effect to reflect annual variation in ecological conditions that may influence the prevalence of T. gondii in the fox population (e.g., climate-related factors, fox density, lemming density). As tissues used to reconstruct diet incorporate dietary information over long timescales (weeks to months), this minimizes the effect of short-term diet variation that could not be captured by using year as a variable in our model.
Stable isotope analysis
To calculate the percentage of fox diet that was derived from selected terrestrial, migratory geese, and aquatic food sources, we used the δ13C values of lemmings, geese, and fish, respectively. As δ13C and δ15N values were very similar for goose muscles and goose eggs22 (t(2) = 0.01, p = 0.49), only goose muscles were used in the analysis. Geese are not present in Nunavik during winter, therefore goose signatures for this period likely represent food cached by foxes in summer for future use. These prey items of foxes were all isotopically distinct (electronic supplementary material, Tables S1, S2). Reassuringly, our isotopic values for lemming in Nunavik were similar to Robillard et al.46, and isotopic signatures of ringed seals (a marine mammal that consumes primarily marine fish) from Nunavik were very similar (1% difference for δ13C and δ15N) (University Laval, unpublished data) to those we described in char and trout.
We corrected red fox samples for isotopic discrimination by using values previously calculated in captive red foxes, i.e., hair: 2.6‰ for δ13C and 3.2‰ for δ15N ratios, muscle tissue: 1.1‰ for δ13C and 3.3‰ for δ15N ratios47. For Arctic foxes, we used values from Lecomte et al.18, i.e., hair: 2.2‰ for δ13C and 3.3‰ for δ15N ratios, muscle tissue: 0.4‰ for δ13C and 1.8‰ for δ15N ratios. We accounted for uncertainty in fractionation estimates in our mixed model analysis for both fox species using estimates from Lecomte et al.18 for muscle (SD of 13C = 0.1‰ and SD of 15N = 0.5‰) and for hair (SD of 13C = 0.4‰ and SD of 15N = 0.6‰). We used SIMMR (R-package SIMMR), a Bayesian stable isotope mixing model, to estimate the proportional contributions of each dietary endpoint (terrestrial, aquatic, and migratory geese) to fox diets. Each model consisted of four Markov Chain Monte Carlo of 1,000,000 iterations, tinned by 100 and with an initial discard of the first 1000 iterations. We performed all statistical analyses (prevalence, risk factors and stable isotope analyses) using R45.
Results
Agreement between ELISA and MC-qPCR
Twenty-six foxes were positive for antibodies to T. gondii on serology and negative for DNA of T. gondii in tissues, one was serologically negative but tissue positive, 66 were positive on both, and 135 were negative on both. There was a statistical difference between serological and molecular results (Χ2 = 21.3, df = 1, p < 0.001, n = 228), but substantial agreement between the two tests (k = 0.74). As a result, for subsequent data analyses, a fox was considered positive if it was positive on either serology or molecular testing.
Detection of T. gondii antibodies and DNA
Antibodies to T. gondii were detected in 40% (CI95%: 34–47) of foxes using ELISA. Toxoplasma gondii DNA was detected in 29% (CI95%: 23–35) of foxes using MC-qPCR. As previously described13, seropositivity and tissue prevalence were higher in Hudson Bay versus Ungava Bay foxes (Table 2).
Risk factors for T. gondii exposure
Linear regression for δ13C and δ15N
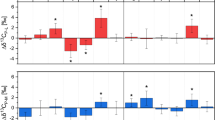
Male foxes had a significantly lower input of δ13C compared to female, and positive foxes had a significantly higher input of δ13C as well as δ15N (Table 3, electronic supplementary material Fig. S1), in both fall and winter diet. The relative contribution of the different dietary endpoints to the diet of foxes according to their infection status and sex can be seen on Fig. 3.
Comparisons of stable isotope (δ13C and δ15N) ratios of dietary endpoints for Nunavik foxes in fall (based on hair) and winter (based on muscle) for sex (A and C), and Toxoplasma gondii infection status (B and D). For both periods, female foxes consumed more aquatic food sources, and positive foxes were more likely to consume migratory geese and aquatic prey.
Logistic regression: fall diet
When fox species were combined, foxes consuming migratory geese or cached eggs in fall were 2 times more likely to be positive for T. gondii (odds ratio: 1.86, CI95%:1.17–2.98, p = 0.009, Table 4). For red foxes, the odds of being positive for T. gondii were 3 times higher in foxes consuming aquatic food sources (odds ratio: 2.95, CI95%:1.43–6.05, p = 0.003, Table 4).
Logistic regression: winter diet
When fox species were combined, foxes consuming migratory geese (most likely from the contribution of cached eggs) in winter were 1.3 times more likely to be positive for T. gondii (odds ratio: 1.27, CI95%:1.02–1.58, p = 0.030, Table 4). As well, red foxes consuming aquatic food sources were 1.3 times more likely to be exposed to T. gondii (odds ratio: 1.29, CI95%:1.03–1.63, p = 0.029, Table 4). The relationship between consuming geese and T. gondii infection was stronger for Arctic foxes in both fall and winter diet (Table 4).
Discussion
This study combines ecological trophic analysis (through stable isotope signatures) with an epidemiological study of infection status of a food borne pathogen in terrestrial mammals, demonstrating that red and Arctic foxes positive for T. gondii were more likely to consume aquatic prey and migratory geese, respectively, and not rodents, their major food source29. Arctic and red foxes were previously hypothesized to be good sentinels for T. gondii circulation in northern ecosystems as they are widespread across northern Canada, exposed at similar rates and routes as humans in many northern regions, and can be exposed to both oocysts shed into the environment by felids and tissue cysts in consumed prey11,13. The likelihood of a fox being positive for T. gondii increased significantly with stable isotope values of δ15N (Table 3). This suggests that positive foxes feed at higher trophic levels, and/or on prey with high values of δ15N that are more likely to be infected with the parasite (i.e., other carnivores). This is consistent with transmission through carnivory or scavenging, supporting our hypothesis that this is a major route of exposure for foxes versus direct oocyst transmission from the environment. Since lemmings are year round resident herbivores (Centre d’études nordiques, unpublished data) in a region where felids are largely absent and less likely to contaminate the environment with oocysts, exposure through this particular prey is less likely; T. gondii infection in rodents has not been reported in Arctic tundra ecosystems5,9.
Although ultimately of terrestrial origin, T. gondii can bioaccumulate in aquatic organisms, playing a significant role in foodborne and waterborne transmission48—a “pathogen pollutant” in aquatic ecosystems. Sporulated oocysts persist in aquatic environments and are resistant to temperature variations49. Red foxes consuming aquatic food sources in fall and winter were more likely to be exposed to the parasite. Spillover to terrestrial wildlife and humans through consumption of aquatic wildlife in the Arctic is often regarded as a possible hypothesis for transmission3,4,50. Coastal areas, in particular, often receive substantial inputs of energy and nutrients from the ocean, and these resources can support large numbers of consumers17. Roth et al.14 found that the stable-carbon isotope ratios of Arctic fox hair indicated the diet was much more marine in winter, probably due to increased access to marine food sources such as carcasses of seals killed by polar bears and/or hunters via sea ice. Killengreen et al.51 also found that red foxes close to the coast in winter had strong isotopic signatures of marine components, and rely on these resources when terrestrial prey became scarce. We found that female foxes had a higher input of δ13C, indicating that female foxes consumed more marine food sources. This may be due to higher metabolic demands for female foxes raising pups, resulting in females staying closer to coastal areas than males, who spend more time dispersing and moving between territories52. Although foxes are known to travel long distances for breeding and dispersal, foxes tend to settle in high-quality habitat patches where prey density is high, and often close to their natal areas53. For this reason, we are relatively confident that foxes sampled in the study were consuming local prey in the months prior to being trapped; i.e., given our wide study area, most juvenile foxes that we sampled would be undergoing natal dispersal54 rather than migrating from outside Nunavik.
Foxes consuming migratory geese for fall diet were twice as likely to be positive for T. gondii, especially Arctic foxes. This is not unexpected given previous epidemiological studies showing that migratory geese (mostly Branta canadensis) in Nunavik harbor T. gondii, that Inuit consuming waterfowl are at higher risk of being exposed to the parasite, and that migratory birds are a likely source of T. gondii exposure for foxes3,25. Foxes in our study area would have access to nesting geese and their eggs, and migratory geese passing through on their way south from May until late September. The highest densities of breeding Canada geese are found in the two main regions of Nunavik where foxes were trapped: coastal lowlands of eastern Hudson Bay and coastal lowlands of southwestern Ungava Bay55. Although Arctic foxes are thought to rely heavily on fluctuating rodent populations, having a regular large influx of birds (and cached eggs) provides foxes with predictable food resources; Arctic foxes are well-known to exhibit hoarding behavior and cached eggs can be heavily utilized during fall and winter months56,57,58. This behavior could explain the higher prevalence of T. gondii in Arctic vs red foxes in our study (Table 2), with greater reliance on these migratory geese.
We observed higher sero- and tissue prevalence in foxes from Hudson Bay (65% and 51%, respectively) compared to Ungava Bay (29% and 20%, respectively). A similar pattern is also seen in people, with a seroprevalence of 56% in Hudson Bay and 37% in Ungava Bay3. In people, this was explained by a higher consumption of marine mammals, fish, and geese in Hudson Bay and Hudson Strait compared to Ungava Bay3,59,60. We hypothesize that the same scenario could be happening in foxes, with those in Hudson Bay having greater access to migratory birds, fish, and carcasses of marine mammals, which could be an important source of T. gondii. As well, contamination of fishes and marine mammals could be related to the geography of the watersheds that irrigate Nunavik. The Hudson watershed originates mostly from subarctic and boreal regions, where lynx could be shedding oocysts in the environment, while the Ungava watershed is restricted to the tundra for the most part3,50,61.
Epidemiological study limitations include disagreement between detection methods for T. gondii: 26 foxes were positive on serology, but DNA was not detected in tissues. This discrepancy is expected and is frequently explained by acute exposure where tissue invasion has not yet occurred, low tissue infection intensity (below the detection limit of the molecular technique), and/or non-uniform distribution of tissue cysts of T. gondii11. As well, false-seropositive results could occur due to the high blood content in heart juice which may have interfered with antibody binding62, or from cross-contamination between samples (less likely since negative controls remained negative). Only one fox was positive for DNA in tissues and negative on serology. This individual could be acutely infected and had not yet developed antibodies, or harbor a senescent infection i.e., antibodies against T. gondii have declined, with tissue cysts persisting in a non-immunogenic state38,63. We acknowledge sampling bias in estimating prevalence using trapped foxes; infection with T. gondii has been linked to increased risk behaviours, such as entering a trap64. Therefore, the proportion of positive animals in this study may overestimate the prevalence of toxoplasmosis in the population, but also ensured that we had an adequate sample size of positive foxes to explore dietary links. Limitations of the trophic ecological aspects of the study include our inability to dictate time of year of fox trapping by community collaborators (fur harvest occurs only in winter), migratory and other movements of wildlife, and the use of stable isotope analysis as an indirect method for determining animal diets. As there is no a priori information available for the diet of foxes in the present study, we exercise caution when interpreting the results since there can be considerable inter- and intra-population variability in diet65. We also acknowledge that we did not sample all possible food sources in our choice of dietary endpoints (e.g., scavenging on caribou carcasses), but instead focused on three most relevant preys for foxes at the local scale.
Conclusion
Our findings support that stable isotope analysis is a powerful tool for tracking food-borne parasite transmission through food webs. We worked with community harvesters and combined epidemiological and ecological methods to unravel the complex transmission of a ubiquitous food-borne pathogen (T. gondii) in a remote environment. While parasites and trophic relationships have been studied before66,67, very few have linked infection status with trophic relationships using stable isotopes68. Our results suggest that non-invasive hair sampling coupled with serology holds promise for in vivo approaches to source attribution of food-borne pathogens, especially in wildlife of conservation significance, and in people. We found that migratory geese and aquatic food sources are potentially a significant contributor to the transmission of T. gondii in wildlife sentinels in northern regions, echoing recent surveys in people in Nunavik3. Such baseline data has never been more important, as the Arctic is experiencing unprecedented temperature and precipitation change69. With increased climate variability and extreme weather events, the ecology and transmission of T. gondii is expected to shift70. The melting of ice and permafrost, in addition to increased precipitation, will likely boost the transport of oocysts in northern ecosystems. As well, warmer temperatures will favor oocyst survival and development71. As the tree line moves northward, the habitat range of lynx and their prey species will likely follow, also possibly affecting fox and human diets in northern ecosystems72. Understanding current trophic relationships and parasite transmission in foxes as a sentinel system will allow us to detect and predict changes in a rapidly warming Arctic, including altered zoonotic risk for northern human populations in the Arctic, who remain intricately linked to wildlife populations and the land.
Data availability
All data relating to this study are available on request from the corresponding author.
References
Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet 363, 1965–1976. https://doi.org/10.1016/S0140-6736(04)16412-X (2004).
Dubey, J. P. Toxoplasmosis of Animals and Humans (CRC Press, 2010).
Ducrocq, J. et al. Epidemiology associated with the exposure to Toxoplasma gondii in Nunavik’s Inuit population using the 2017 Qanuilirpitaa cross-sectional health survey. Zoonoses Public Health 68, 803–814. https://doi.org/10.1111/zph.12870 (2021).
Messier, V. et al. Seroprevalence of Toxoplasma gondii among Nunavik Inuit (Canada). Zoonoses Public Health 56, 188–197. https://doi.org/10.1111/j.1863-2378.2008.01177.x (2009).
Prestrud, K. W. et al. Serosurvey for Toxoplasma gondii in arctic foxes and possible sources of infection in the high Arctic of Svalbard. Vet. Parasitol. 150, 6–12. https://doi.org/10.1016/j.vetpar.2007.09.006 (2007).
Arkush, K. D. et al. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis). Int. J. Parasitol. 33, 1087–1097. https://doi.org/10.1016/s0020-7519(03)00181-4 (2003).
Marino, A. M. F. et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health 66, 826–834. https://doi.org/10.1111/zph.12630 (2019).
Baker, T., Flaig, J., Shillingford, M., Swain, L. & Wagner, M. Ice road vets: Perspectives on the role of veterinarians in northern community health. Can. Vet. J. 59, 668–672 (2018).
Elmore, S. A., Samelius, G., Fernando, C., Alisauskas, R. T. & Jenkins, E. J. Evidence for Toxoplasma gondii in migratory vs. nonmigratory herbivores in a terrestrial arctic ecosystem. Can. J. Zool. 93, 671–675. https://doi.org/10.1139/cjz-2015-0078 (2015).
Jenkins, E. J. et al. Tradition and transition: Parasitic zoonoses of people and animals in Alaska, Northern Canada, and Greenland. In Advances in Parasitology (ed. Rollinson, D) 33–204 (Academic Press, 2013).
Bachand, N. et al. Foxes (Vulpes vulpes) as sentinels for parasitic zoonoses, Toxoplasma gondii and Trichinella nativa, in the northeastern Canadian Arctic. Int. J Parasitol. Parasites Wildl. 7, 391–397. https://doi.org/10.1016/j.ijppaw.2018.10.003 (2018).
Bouchard, É., Jokelainen, P., Sharma, R., Fenton, H. & Jenkins, E. J. Toxoplasmosis in northern regions. In Arctic One Health: Challenges for Northern Animals and People (ed. Tryland, M.) 297–314 (Springer International Publishing, 2022).
Bouchard, É. et al. Are foxes (Vulpes spp.) good sentinel species for Toxoplasma gondii in northern Canada?. Parasite Vect. 15, 115. https://doi.org/10.1186/s13071-022-05229-3 (2022).
Roth, J. D. Temporal variability in arctic fox diet as reflected in stable-carbon isotopes; the importance of sea ice. Oecologia 133, 70–77. https://doi.org/10.1007/s00442-002-1004-7 (2002).
Savory, G. A., Hunter, C. M., Wooller, M. J. & O’Brien, D. M. Anthropogenic food use and diet overlap between red foxes (Vulpes vulpes) and arctic foxes (Vulpes lagopus) in Prudhoe Bay, Alaska. Can. J. Zool. 92, 657–663. https://doi.org/10.1139/cjz-2013-0283 (2014).
Hoekstra, P. F. et al. Organochlorine contaminant and stable isotope profiles in Arctic fox (Alopex lagopus) from the Alaskan and Canadian Arctic. Environ. Pollut. 122, 423–433. https://doi.org/10.1016/S0269-7491(02)00310-X (2003).
Roth, J. D. Variability in marine resources affects arctic fox population dynamics. J. Anim. Ecol. 72, 668–676. https://doi.org/10.1046/j.1365-2656.2003.00739.x (2003).
Lecomte, N. et al. Intrapopulation variability shaping isotope discrimination and turnover: Experimental evidence in arctic foxes. PLoS One 6, e21357. https://doi.org/10.1371/journal.pone.0021357 (2011).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506. https://doi.org/10.1016/0016-7037(78)90199-0 (1978).
DeNiro, M. J. & Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351. https://doi.org/10.1016/0016-7037(81)90244-1 (1981).
Kelly, J. F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 78, 1–27. https://doi.org/10.1139/z99-165 (2000).
Bonin, M., Dussault, C., Taillon, J., Lecomte, N. & Cote, S. D. Combining stable isotopes, morphological, and molecular analyses to reconstruct the diet of free-ranging consumers. Ecol. Evol. 10, 6664–6676. https://doi.org/10.1002/ece3.6397 (2020).
Boecklen, W. J., Yarnes, C. T., Cook, B. A. & James, A. C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 42, 411–440. https://doi.org/10.1146/annurev-ecolsys-102209-144726 (2011).
Sandström, C. A. et al. Latitudinal variability in the seroprevalence of antibodies against Toxoplasma gondii in non-migrant and Arctic migratory geese. Vet. Parasitol. 194, 9–15. https://doi.org/10.1016/j.vetpar.2012.12.027 (2013).
Bachand, N. et al. Serological and molecular detection of Toxoplasma gondii in terrestrial and marine wildlife harvested for food in Nunavik, Canada. Parasit Vect. 12, 155. https://doi.org/10.1186/s13071-019-3408-9 (2019).
Wootton, B., Durkalec, A. & Ashley, S. Nunavik Regional Impact Analysis. In Centre for Alternative Wastewater Treatment, Fleming College, Lindsay, Ontario 62 (2008).
Bercuson, D. J., Krueger, R. R., Hall, R. D., Morton, W. L. & Nicholson, N. L. Encyclopedia Britannica (2021, accessed 8 Apr 2021). https://www.britannica.com/place/Canada.
MFFP. Zones de végétation et domaines bioclimatiques du Québec, Ministère des Forêts, de la Faune et des Parcs, Gouvernement du Québec. https://mffp.gouv.qc.ca/forets/inventaire/inventaire-zones-carte.jsp (2019).
Chester, S. The Arctic Guide: Wildlife of the Far North (Princeton University Press, 2016).
Anthony, R. M., Barten, N. L. & Seiser, P. E. Foods of arctic foxes (Alopex lagopus) during winter and spring in western Alaska. J. Mammal. 81, 820–828. https://doi.org/10.1644/1545-1542(2000)081%3c0820:foafal%3e2.3.co;2 (2000).
Jung, T. et al. Red fox (Vulpes vulpes) scavenging on the spring sea ice: Potential implications for Arctic food webs. Can. Field-Nat. 134, 144–146. https://doi.org/10.22621/cfn.v134i2.2375 (2020).
Schmidt, N. M. et al. Spatio-temporal patterns in arctic fox (Vulpes alopex) diets revealed by molecular analysis of scats from Northeast Greenland. Polar Sci. 32, 100838. https://doi.org/10.1016/j.polar.2022.100838 (2022).
Gisbert, A. I. et al. Pork as a source of transmission of Toxoplasma gondii to humans: A parasite burden study in pig tissues after infection with different strains of Toxoplasma gondii as a function of time and different parasite stages. Int. J. Parasitol. 48, 555–560. https://doi.org/10.1016/j.ijpara.2017.12.009 (2018).
Juránková, J. et al. Brain is the predilection site of Toxoplasma gondii in experimentally inoculated pigs as revealed by magnetic capture and real-time PCR. Food Microbiol. 38, 167–170. https://doi.org/10.1016/j.fm.2013.08.011 (2014).
Koethe, M. et al. Quantitative detection of Toxoplasma gondii in tissues of experimentally infected turkeys and in retail turkey products by magnetic-capture PCR. Food Microbiol. 52, 11–17. https://doi.org/10.1016/j.fm.2015.06.005 (2015).
Sharma, R. et al. Risk factors and prevalence of antibodies for Toxoplasma gondii in diaphragmatic fluid in wolverines (Gulo gulo) from the Northwest Territories, Canada. Food Waterborne Parasitol. 15, e00056. https://doi.org/10.1016/j.fawpar.2019.e00056 (2019).
Sharma, R., Parker, S., Al-Adhami, B., Bachand, N. & Jenkins, E. Comparison of tissues (heart vs. brain) and serological tests (MAT, ELISA and IFAT) for detection of Toxoplasma gondii in naturally infected wolverines (Gulo gulo) from the Yukon, Canada. Food Waterborne Parasitol. 15, 0046. https://doi.org/10.1016/j.fawpar.2019.e00046 (2019).
Opsteegh, M. et al. Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. Int. J. Food Microbiol. 139, 193–201. https://doi.org/10.1016/j.ijfoodmicro.2010.02.027 (2010).
Reiling, S. J. & Dixon, B. R. Toxoplasma gondii: How an Amazonian parasite became an Inuit health issue. Can. Commun. Dis. Rep. 45, 183–190. https://doi.org/10.4745/ccdr.v45i78a03 (2019).
Chesemore, D. L. Notes on the pelage and priming sequence of Arctic foxes in northern Alaska. J. Mammal. 51, 156–159. https://doi.org/10.2307/1378547 (1970).
Rau, G. H., Ainley, D. G., Bengtson, J. L., Torres, J. J. & Hopkins, T. L. 15N/14N and 13C/12C in Weddell Sea birds, seals, and fish: Implications for diet and trophic structure. Mar. Ecol. Prog. Ser. 84, 1–8 (1992).
Bonin, M., Dussault, C. & Côté, S. D. Increased trophic position of black bear (Ursus americanus) at the northern fringe of its distribution range. Can. J. Zool. 98, 127–133. https://doi.org/10.1139/cjz-2019-0062 (2020).
Sergeant, E. S. G. Epitools Epidemiological Calculators. Ausvet. http://epitools.ausvet.com.au (2018).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
R-Core-Team. R: A language and environment for statistical computing. Vienna, Austria. https://www.R-project.org/ (2019).
Robillard, A., Gauthier, G., Therrien, J. F. & Bety, J. Linking winter habitat use, diet and reproduction in snowy owls using satellite tracking and stable isotope analyses. Isotopes Environ Health Stud. 57, 166–182. https://doi.org/10.1080/10256016.2020.1835888 (2021).
Roth, J. D. & Hobson, K. A. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: Implications for dietary reconstruction. Can. J. Zool. 78, 848–852 (2000).
Shapiro, K. et al. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 15, e00049. https://doi.org/10.1016/j.fawpar.2019.e00049 (2019).
Lindsay, D. S. & Dubey, J. P. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 95, 1019–1020. https://doi.org/10.1645/GE-1919.1 (2009).
Simon, A., Poulin, M. B., Rousseau, A. N. & Ogden, N. H. Fate and transport of Toxoplasma gondii oocysts in seasonally snow covered watersheds: A conceptual framework from a melting snowpack to the Canadian arctic coasts. Int. J.. Environ Res Public Health. 10, 994–1005. https://doi.org/10.3390/ijerph10030994 (2013).
Killengreen, S. T. et al. The importance of marine vs human-induced subsidies in the maintenance of an expanding mesocarnivore in the arctic tundra. J. Anim. Ecol. 80, 1049–1060. https://doi.org/10.1111/j.1365-2656.2011.01840.x (2011).
Walton, Z. et al. Moving far, staying close: Red fox dispersal patterns revealed by SNP genotyping. Conserv. Genet. 22, 249–257. https://doi.org/10.1007/s10592-021-01332-7 (2021).
Angerbjörn, A., Hersteinsson, P. & Tannerfeldt, M. Arctic foxes: Consequences of resource predictability in the Arctic fox—two life history strategies. In The Biology and Conservation of Wild Canids (eds. David W. et al.) 163–172 (Oxford Academic, 2004).
Gravel, R., Lai, S. & Berteaux, D. Long-term satellite tracking reveals patterns of long-distance dispersal in juvenile and adult Arctic foxes (Vulpes lagopus). R. Soc. Open Sci. 10, 220729. https://doi.org/10.1098/rsos.220729 (2023).
Cotter, R. C. et al. Breeding biology of Atlantic population Canada geese in Nunavik, Northern Québec. Arctic 66, 301–311. https://doi.org/10.14430/arctic4309 (2013).
Samelius, G., Alisauskas, R. T., Hobson, K. A. & Lariviere, S. Prolonging the arctic pulse: Long-term exploitation of cached eggs by arctic foxes when lemmings are scarce. J Anim Ecol. 76, 873–880. https://doi.org/10.1111/j.1365-2656.2007.01278.x (2007).
Samelius, G. & Alisauskas, R. T. Foraging patterns of Arctic foxes at a large Arctic goose colony. Arctic. 53, 279–288. https://doi.org/10.14430/arctic858 (2000).
Careau, V., Giroux, J.-F. & Berteaux, D. Cache and carry: Hoarding behavior of arctic fox. Behav. Ecol. Sociobiol. 62, 87–96. https://doi.org/10.1007/s00265-007-0441-z (2007).
Blanchet, C. & Rochette, L. Nutrition and Food Consumption among the Inuit of Nunavik. Nunavik Inuit Health Survey 2004, Qanuippitaa? How are we? In Quebec: Institut national de santé publique du Québec (INSPQ) & Nunavik Regional Board of Health and Social Services (NRBHSS). (2008).
Ducrocq, J., Lévesque, B., Lemire, M., de Serres, G. Zoonotic and Gastrointestinal Diseases. Nunavik Inuit Health Survey 2017 Qanuilirpitaa? How are we now? In Quebec: Nunavik Regional Board of Health and Social Services (NRBHSS) & Institut national de santé publique du Québec (INSPQ) (2021).
Québec, G. d. Rapport sur l’état de l’eau et des écosystèmes aquatiques au Québec: De l’eau du nord au sud et d’est en ouest. http://www.environnement.gouv.qc.ca/rapportsurleau/plan.htm (2020).
Mecca, J. N., Meireles, L. R. & de Andrade, H. F. Quality control of Toxoplasma gondii in meat packages: Standardization of an ELISA test and its use for detection in rabbit meat cuts. Meat Sci. 88, 584–589. https://doi.org/10.1016/j.meatsci.2011.01.016 (2011).
Robert-Gangneux, F. & Darde, M. L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. https://doi.org/10.1128/CMR.05013-11 (2012).
Webster, J. P. The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology 109, 583–589. https://doi.org/10.1017/s0031182000076460 (1994).
Phillips, D. L. et al. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 92, 823–835. https://doi.org/10.1139/cjz-2014-0127 (2014).
Wilson, A. G., Lapen, D. R., Mitchell, G. W., Provencher, J. F. & Wilson, S. Interaction of diet and habitat predicts Toxoplasma gondii infection rates in wild birds at a global scale. Glob. Ecol. Biogeogr. 29, 1189–1198. https://doi.org/10.1111/geb.13096 (2020).
Sabadel, A. J. M., Stumbo, A. D. & MacLeod, C. D. Stable-isotope analysis: A neglected tool for placing parasites in food webs. J. Helminthol. 2018, 1–7. https://doi.org/10.1017/S0022149X17001201 (2018).
Cabezón, O. et al. Toxoplasma gondii Infection in seagull chicks is related to the consumption of freshwater food resources. Plos One 11, e0150249. https://doi.org/10.1371/journal.pone.0150249 (2016).
Bush, E. & Lemmen, D. S. Canada’s changing climate report; Government of Canada, Ottawa, ON (2019).
Patz, J. A., Graczyk, T. K., Geller, N. & Vittor, A. Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 30, 1395–1405. https://doi.org/10.1016/s0020-7519(00)00141-7 (2000).
Lindsay, D. S., Blagburn, B. L. & Dubey, J. P. Survival of nonsporulated Toxoplasma gondii oocysts under refrigerator conditions. Vet. Parasitol. 103, 309–313. https://doi.org/10.1016/s0304-4017(01)00554-4 (2002).
Lenoir, J., Gégout, J. C., Marquet, P. A., de Ruffray, P. & Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768. https://doi.org/10.1126/science.1156831 (2008).
Acknowledgements
We thank trappers and community coordinators, especially Elena Berthe, Eddie Kumarluk, and Lasarusie Tukai. We also thank Mark Basterfield, Frankie Jean-Gagnon, and Ellen Avard from Makivvik Corporation for field logistical support. We are also thankful to Brent Wagner, Champika Fernando, Michelle Sniatynski, Pratap Kafle, Jonathan Gagnon, and Marie-Claude Martin for laboratory analyses. We are grateful to the personnel of the Faculté de Médecine Vétérinaire and the Centre québécois sur la santé des animaux sauvages (CQSAS), the Nunavik Research Centre (Makivvik Corporation), and regional and local Nunavimmi Umajulivijiit Katujaqatigininga (LNUK and RNUK) who were key to the success of this study. We acknowledge funding from the ArcticNet Networks of Centres of Excellence (P63), Natural Sciences and Engineering Research Council of Canada and Northern Research Supplement (RGPIN-2012-386666, RGPNS-2012-424278, RGPIN-2018-04900, NRS-2018-517969), Polar Knowledge Canada (NST-1718-0012), and Weston Family Foundation.
Author information
Authors and Affiliations
Contributions
É.B., E.J., and P.L. conceived and designed the study; E.J. and P.L. supervised the work; É.B. and M.B. collected foxes and performed necropsies; M.B., G.-G.G., A.S., P.L., and E.J. helped in sample logistics; É.B., M.B., R.S., G.-G.G., and A.H.-O. performed laboratory analyses; É.B. and M.B. performed statistical analysis; É.B. and M.B. wrote the first draft. All authors contributed critically to the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bouchard, É., Bonin, M., Sharma, R. et al. Use of stable isotopes to reveal trophic relationships and transmission of a food-borne pathogen. Sci Rep 14, 2812 (2024). https://doi.org/10.1038/s41598-024-53369-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53369-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.